Is Retifanlimab approved by the FDA?
Retifanlimab, marketed under the brand name Zynyz, was approved by the FDA on March 22, 2023, for the treatment of adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). This approval was granted under accelerated approval based on the tumor response rate and duration of response, with continued approval contingent upon the verification and description of clinical benefits in confirmatory trials.
Usage and Dosage
Retifanlimab is administered as an intravenous infusion over 30 minutes, typically every 4 weeks. The usual dosage for treating Merkel cell carcinoma is 500 mg. The treatment continues until disease progression, unacceptable toxicity, or up to 24 months.
Side Effects
Common side effects of retifanlimab may include:
- Tiredness
- Itching, rash
- Fever
- Muscle and bone pain
- Nausea, diarrhea
Serious side effects requiring immediate medical attention include:
- Cough, shortness of breath, chest pain
- Irregular heartbeat
- Confusion, drowsiness, memory problems, changes in mood or behavior
- Severe muscle pain or weakness, muscle cramps
- Severe stomach pain or tenderness, diarrhea, bloody or tarry stools
- Liver and kidney problems
- Hormonal disorders
Some side effects may occur during the injection, such as dizziness, nausea, light-headedness, itching, sweating, headache, chest tightness, back pain, trouble breathing, or swelling in the face.
Warnings and Precautions
Before starting retifanlimab, it is important to inform your doctor if you have:
- Immune system problems such as ulcerative colitis, Crohn's disease, or lupus
- A nervous system condition, such as myasthenia gravis or Guillain-Barré syndrome
- Received an organ transplant
- Received or plan to receive a stem cell transplant that uses donor stem cells
- Received radiation treatment to your chest area
Women of childbearing potential should have a negative pregnancy test before starting treatment and use effective birth control during treatment and for at least 4 months after the last dose. Breastfeeding is not recommended during treatment and for at least 4 months after the last dose.
Interactions
Retifanlimab can interact with other medications, including prescription and over-the-counter medicines, vitamins, and herbal products. It is crucial to inform your healthcare provider about all the medications you are currently taking to avoid potential interactions.
Monitoring
Patients undergoing treatment with retifanlimab will require frequent blood tests to monitor liver function and other parameters. Any missed doses should be discussed with the healthcare provider to determine the next steps.
Retifanlimab offers a promising treatment option for adults with advanced Merkel cell carcinoma. Always follow your healthcare provider's instructions and report any side effects or concerns promptly.
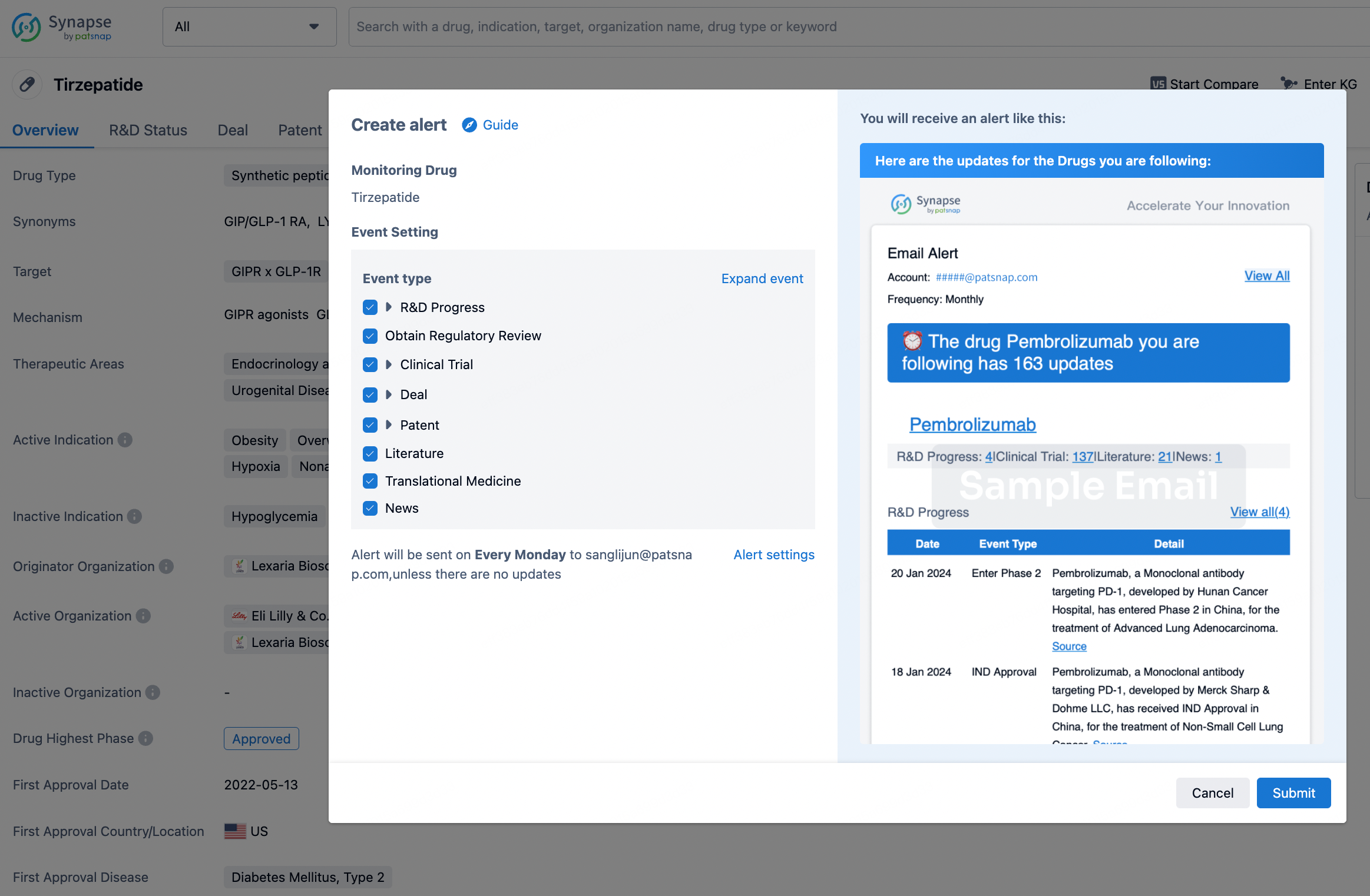
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!