MICA/B Antibody DM919 Enters Phase 1 Trial for Advanced Solid Tumors by D2M Biotherapeutics
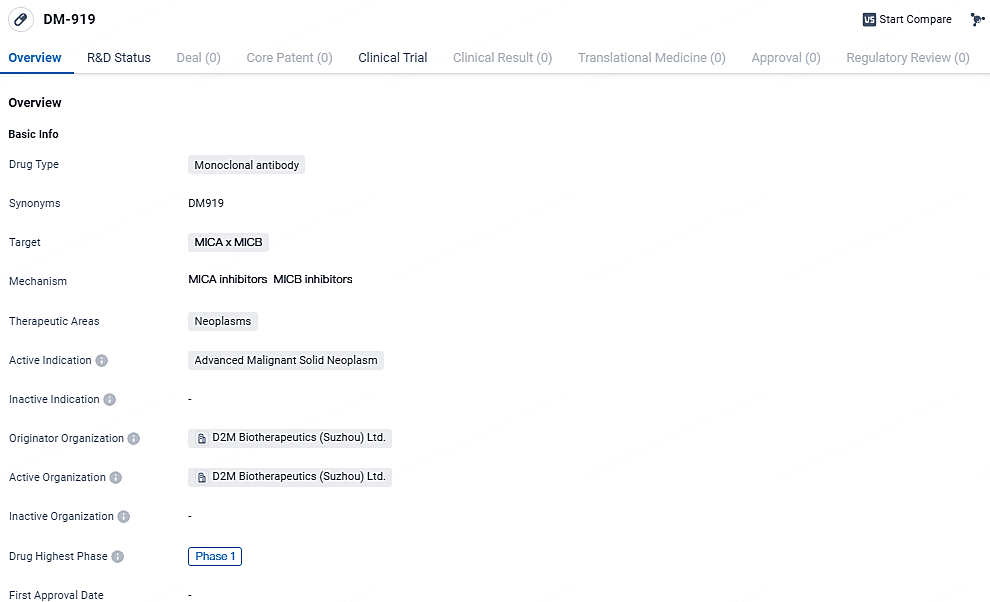
D2M Biotherapeutics, Inc. has initiated the dosing of the initial participant in a Phase 1 open-label study, which includes both dose escalation and expansion phases, focusing on DM919. This innovative monoclonal antibody targets MICA/B and is designed to enhance and stimulate the anti-tumor activities of T cells and natural killer cells in treating advanced solid tumors.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This initial phase 1 study, which is a multicenter, first-in-human investigation involving dose escalation and expansion, aims to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and therapeutic efficacy of DM919 when used alone and coupled with anti-PD1 therapy in individuals suffering from advanced or metastatic solid tumors.
Dr. Nan Bing, MD., Ph.D., a co-founder and CEO of D2M, elaborated, “MICA/B targeted therapy introduces a cutting-edge approach to cancer care. It disrupts the MICA/B mediated immune evasion seen in numerous tumor forms and holds promise for treating certain cancers that show low MHC-I expression, which are commonly resistant to existing therapies targeting immune checkpoints.”
Dr. Sen remarked, “The distinctive biological characteristics of DM919 could revitalistically aid both innate and adaptive immune systems in fighting cancers. We are enthusiastic about exploring its capabilities as a standalone treatment and when paired with anti-PD1 therapy.”
DM919 is a humanized, MICA/B-specific, IgG1 monoclonal antibody crafted to inhibit the cleavage of MICA and MICB from tumor cells. Predominantly overexpressed in cancerous cells, MICA/B acts as stress-induced ligands that are recognizable by both innate and adaptive immune systems via NKG2D receptors. By halting the shedding process of MICA/B, DM919 hinders the immune evasion mechanism of the tumor cells, thereby reactivating and boosting the NKG2D-dependent activation of T-cells and NK cells within the tumor environment, augmenting anti-cancer effects.
In preclinical trials, DM919 exhibited notable antitumor properties as a singular therapeutic agent across various tumor models. When combined with an anti-PD1 agent, it also showed extensive synergistic effects in combatting tumors.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
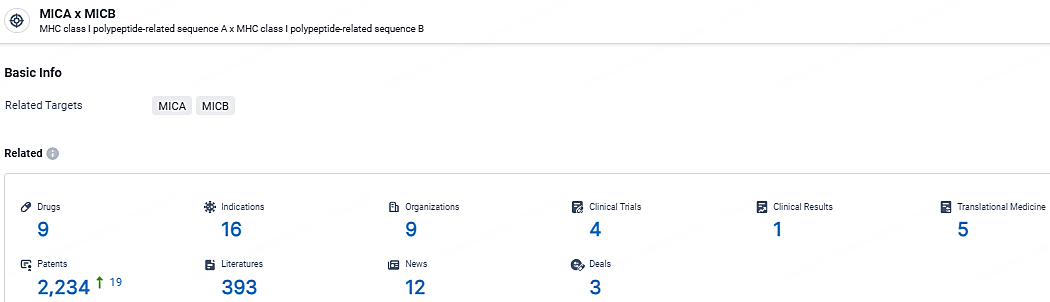
According to the data provided by the Synapse Database, As of April 22, 2024, there are 9 investigational drugs for the MICA/B target, including 16 indications, 9 R&D institutions involved, with related clinical trials reaching 4, and as many as 2234 patents.
DM-919 is a monoclonal antibody drug developed by D2M Biotherapeutics (Suzhou) Ltd. It targets MICA x MICB and is being developed for the treatment of advanced malignant solid neoplasms. The drug has reached Phase 1 globally and has received IND approval in China.