Cidara Therapeutics Retakes Global Rights to CD388, Initiates $240M Funding Drive
Cidara Therapeutics, Inc., a biotech firm leveraging its unique Cloudbreak technology to create immunotherapies based on drug-Fc conjugates for serious illnesses, has declared its entry into a final agreement with Johnson & Johnson®. Under this agreement, Cidara will regain the sole worldwide rights to develop and market CD388, currently being developed to prevent all strains of influenza A and B.
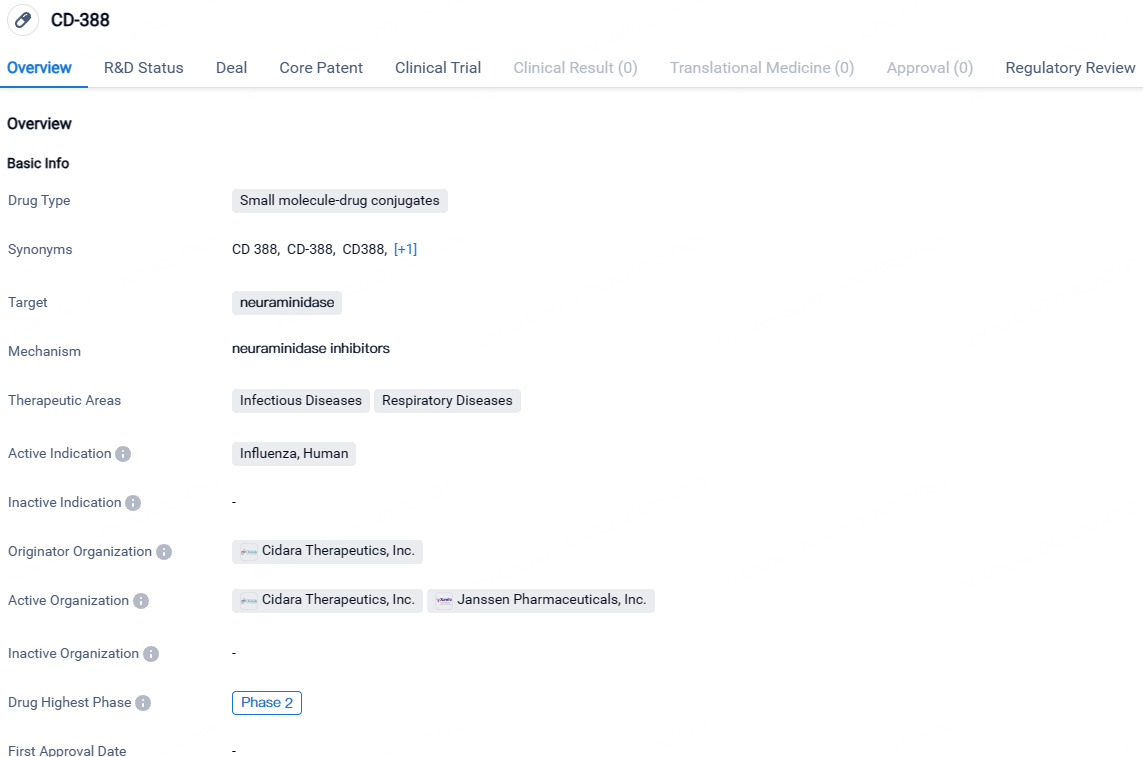
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Concurrent with the acquisition, Cidara closed a definitive agreement for the sale of preferred Simultaneously with its acquisition, Cidara finalized a definitive agreement to sell preferred stock through a private placement spearheaded by RA Capital Management. This offering also saw notable contributions from Bain Capital Life Sciences, Biotech Value Fund, and Canaan Partners. The private placement secured $240 million in gross proceeds for Cidara, earmarked for the advancement of CD388 as a broad-spectrum preventative for both seasonal and pandemic influenza types A and B. This initiative will kick off with a Phase 2b clinical trial during the upcoming flu season in the Northern Hemisphere. The funds from this initiative will also cover the initial payment for a contract with Johnson & Johnson and are anticipated to extend the financial runway following the initial results from the CD388 Phase 2b study.
CD388, a novel long-acting antiviral therapy developed by Cidara, was licensed exclusively to Johnson & Johnson worldwide in April 2021 under a collaborative agreement. In September 2023, Johnson & Johnson issued a Proceed Notice to Cidara, along with an associated milestone payment for CD388, and commenced the process of transferring its contractual rights and responsibilities to a different company.
With this new financing in place, RA Capital Management's Laura Tadvalkar, Ph.D., along with Ryan Spencer and James Merson, Ph.D., have joined Cidara’s board of directors. Concurrently, David Gollaher, Ph.D., and Timothy Franson, M.D. have resigned from the board. "I am grateful to David and Tim for their significant contributions, which have paved the way for this significant milestone for Cidara," commented Jeffrey Stein, Ph.D., President and CEO of Cidara.
Cidara will now take over all future responsibilities related to the development, production, and marketing of CD388. In return for regaining exclusive worldwide development and marketing rights for CD388, Johnson & Johnson received a one-time upfront payment of $85 million from Cidara, with potential for future additional payments based on development, regulatory, and sales milestones.
Dr. Tadvalkar expressed enthusiasm about the progress of CD388, stating, "Watching CD388 develop has been incredibly engaging. As investors indifferent to development stages, we are thrilled to have facilitated this buy-back and financing, enabling us to use our RAVen incubator resources to bolster Cidara’s growth."
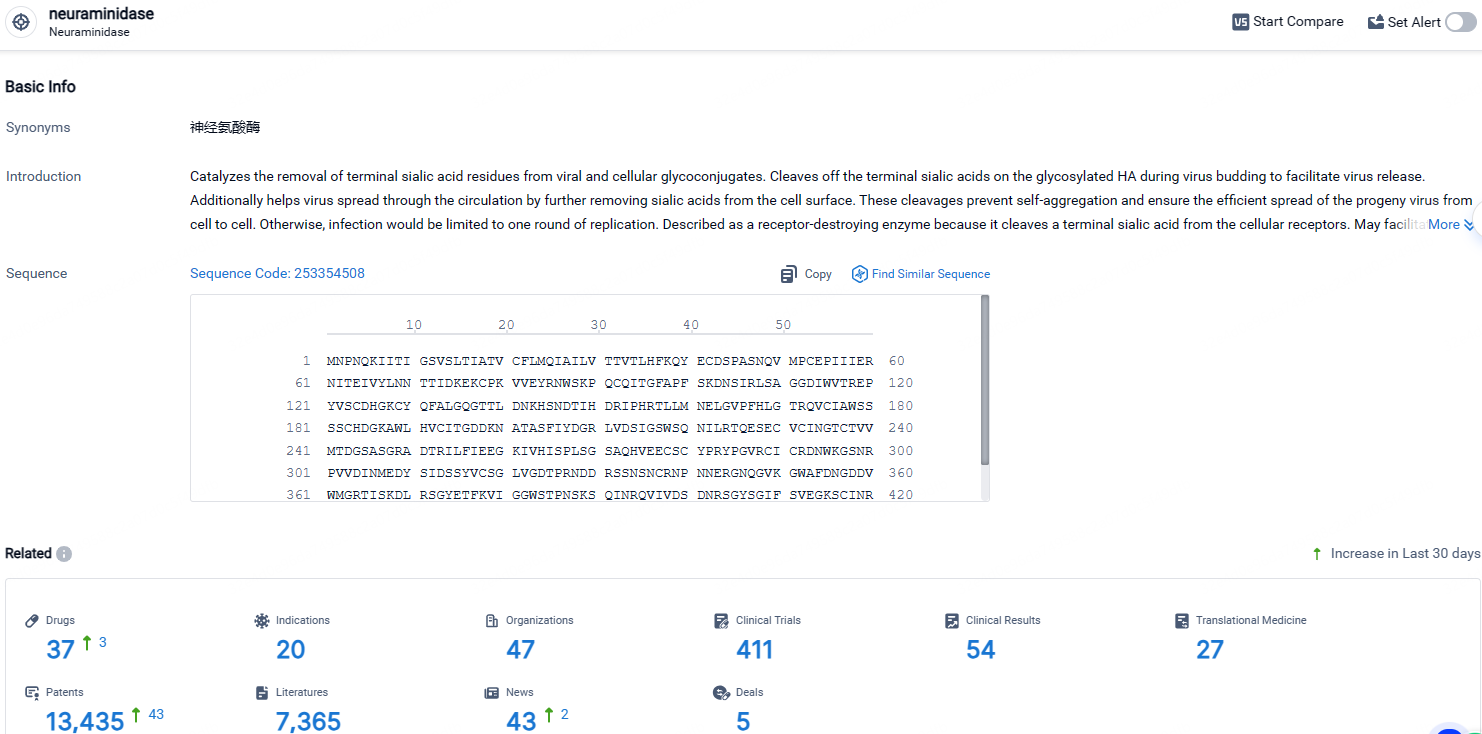
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 28, 2024, there are 37 investigational drugs for the neuraminidase targets, including 20 indications, 47 R&D institutions involved, with related clinical trials reaching 411, and as many as 13435 patents.
CD-388 is a small molecule-drug conjugate that targets neuraminidase and is being developed by Cidara Therapeutics, Inc. for the treatment of influenza in humans. The drug is currently in Phase 2 of its development and has been granted Fast Track designation, indicating its potential to address an unmet medical need. CD-388 falls under the therapeutic areas of infectious diseases and respiratory diseases, highlighting its focus on treating conditions related to infections and the respiratory system.