Circular RNA: A Gene Therapy Alternative to mRNA
Circular RNA (circRNA) is a type of single-stranded RNA with a closed loop structure, initially found in HDV and plant viruses. circRNA is a class of non-coding RNAs ubiquitously distributed in eukaryotic cells. CircRNA can act as a potent adjuvant to induce specific T and B cell responses, and can inhibit the establishment and growth of tumors. Its circular structure protects it from degradation by nucleases, resulting in high stability. It is widely accepted that the generation of circular RNA exploits the splicing mechanism used by eukaryotic cells to generate mRNA, forming circular RNA through the same enzymatic system via back-splicing. In certain circumstances, circularization can be promoted by bringing together the ends of a particular exon, such as through intronic sequences containing palindromic or near-palindromic sequences hybridizing, or through RNA Binding Proteins (RBPs) binding on introns at the ends of exons. From an evolutionary perspective, the formation of circular RNA can be seen as a serendipity of back-splicing, and has gradually given rise to mechanisms that actively produce new forms of back-splicing.
Circular RNA (circRNA) has broad application prospects. An increasing number of studies have found that circRNA is associated with various diseases such as infectious diseases, rare diseases, hematological disorders, autoimmune diseases, and tumors.
Nucleic acid drugs have heralded the third wave of drug revolution following small molecule drugs and antibody drugs, and, with the momentum from mRNA vaccine industry, circRNA technology is rapidly emerging as the next generation RNA therapy. Endogenous circRNAs can serve as new drug targets or disease diagnosis biomarkers, while artificially prepared circRNAs can target various elements and function within cells. Evidently, circRNA will become a key technology for the global bio-economy in the future. Numerous new circRNA therapy enterprises have made breakthroughs in areas such as COVID-19 vaccine, CAR-T, protein replacement therapy, among others. Preparing circRNA in vitro is a critical premise for future advancements in scientific research, clinical developments, and industrialization.
Circular RNA Competitive Landscape
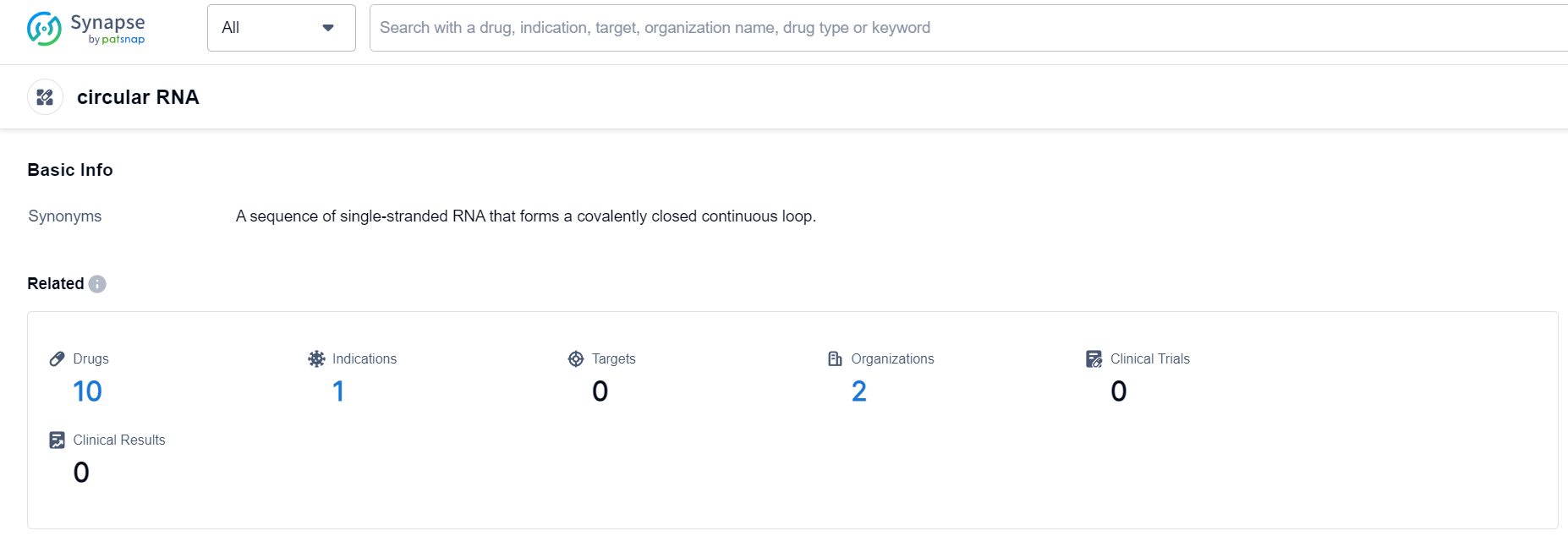
According to Patsnap Synapse, as of 24 Sep 2023, there are a total of 10 circular RNA drugs worldwide, from 2 organizations, covering 0 targets, 1 indications, and conducting 0 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
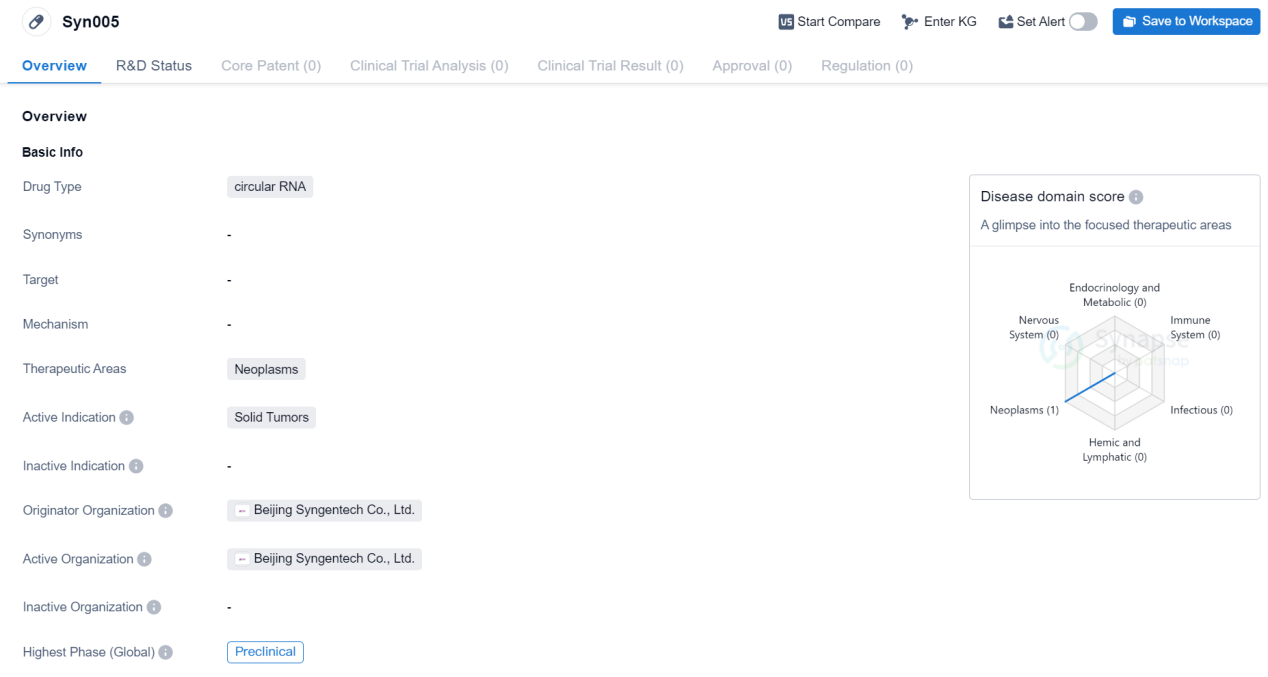
PreClinical Circular RNA Medicinal: Syn005
Syn005 is a circular RNA drug being developed by Beijing Syngentech Co., Ltd. It falls under the therapeutic area of neoplasms, specifically targeting solid tumors. As of now, the drug is in the preclinical phase globally.
Circular RNA drugs are a relatively new and promising class of therapeutics in the field of biomedicine. Unlike linear RNA molecules, circular RNAs form a closed loop structure, which makes them more stable and resistant to degradation. This stability allows circular RNAs to have a longer half-life in the body, potentially leading to improved drug efficacy and reduced dosing frequency.
The specific mechanism of action of Syn005 is not provided in the given information. However, it is likely that the drug is designed to target and inhibit specific molecular pathways or proteins involved in the growth and survival of solid tumors. By doing so, Syn005 may have the potential to slow down or even halt the progression of these tumors.
Being in the preclinical phase means that Syn005 is still undergoing extensive laboratory testing and evaluation. This phase involves in vitro and in vivo studies to assess the drug's safety, efficacy, and pharmacokinetics. If the results from these preclinical studies are promising, Syn005 may progress to clinical trials, where it will be tested in humans to further evaluate its safety and effectiveness.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As an early-stage drug candidate, Syn005 is still a long way from being approved and available for patient use. However, its development in the field of circular RNA therapeutics for solid tumors shows promise and highlights the ongoing efforts to discover innovative treatments for neoplasms. Further research and development will be necessary to determine the full potential of Syn005 and its eventual impact on cancer treatment.