Clover Reveals Encouraging Early Data for SCB-1019, a Dual RSV Immunization, in First Group of Young Adults
Announcing a major advancement, Clover Biopharmaceuticals, Ltd. , a worldwide biotech firm actively engaged in harnessing the potential of novel immunizations to enhance global health and save lives, recently reported encouraging early-stage results for the younger adult population in their Phase I study. These findings concern the immunogenicity and safety profiles of their investigational vaccine, SCB-1019. This vaccine is a dual-component candidate utilizing the company's proprietary Trimer-Tag technology and is designed to target RSV (respiratory syncytial virus) through a stabilized prefusion F (PreF)-Trimer structure.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"We find the advancements and the encouraging initial findings from Phase I of our dual RSV PreF vaccine prospect, built upon our reputable Trimer-Tag technology, to be quite promising. These findings have showcased widespread potent neutralizing antibody activities against both RSV-A and RSV-B strains," commented Joshua Liang, the CEO and Member of the Board of Directors at Clover.
He further stated, "Marking a milestone as China’s initial venture into RSV PreF vaccine clinical trials, and the pioneer in yielding clinical outcome data, we are eager to examine more detailed Phase I clinical results tailored for our preliminary key demographic—older adults—expected in the latter half of 2024."
In this examination, third-party labs carried out the neutralizing assessments for RSV-A and RSV-B employing clinically validated procedures and made use of the NIBSC 16/284 standard reference sera. Test readings were denoted in international units per milliliter (IU/mL).
Comparative immunogenicity data from Clover suggest that the immunogenic response towards both RSV-A and RSV-B could be on par with or potentially better than other protein subunit RSV PreF vaccinations. This supports Clover's dual RSV-A/B vaccine strategy, especially since some prior single-descent RSV-A inoculations demonstrated diminished immunogenic reactions and/or protection against RSV-B.
The findings further affirm the presence of SCB-1019's PreF antigens in their stable pre-fusogenic, trimolecular conformation, a fact that is also corroborated by preliminary immunogenicity studies indicating substantial spikes in neutralizing antibody levels that competitively inhibit Site Ø. Furthermore, the inoculation of SCB-1019 in the initial group of young adults has been marked by the absence of significant safety or tolerability issues, paving the way for the upcoming participation of older adults in the ongoing Phase I trial.
The ongoing Phase I study being conducted in Australia is an organized, placebo-influenced investigation aimed at determining the tolerability, safety measures, and immunogenicity of SCB-1019 across various dosages and formulations amongst younger and senior participants. It is anticipated that additional findings on the safety and immunogenicity within the elder group will be available by the second semester of 2024.
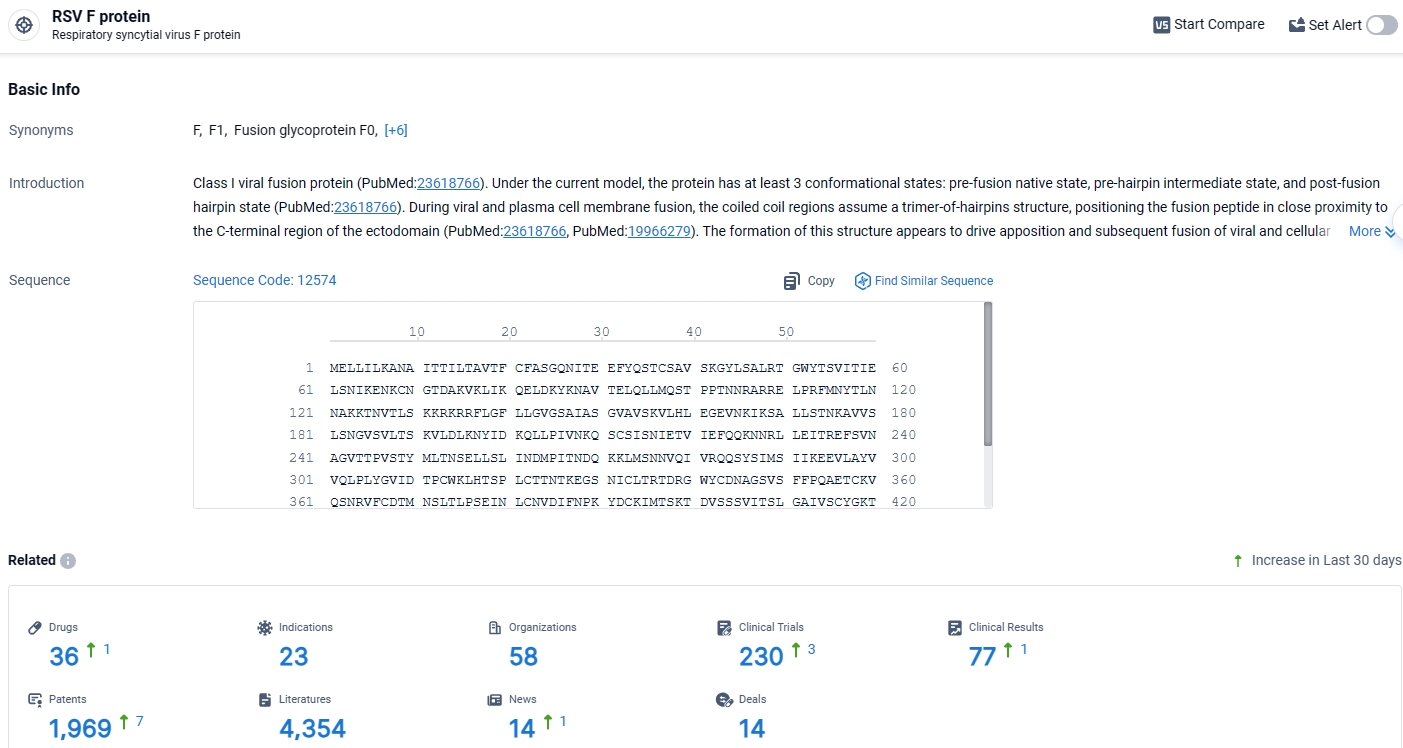
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 9, 2024, there are 36 investigational drugs for the RSV F protein target, including 23 indications, 58 R&D institutions involved, with related clinical trials reaching 230, and as many as 1969 patents.
SCB-1019 developed by Clover Biopharmaceuticals is a vaccine targeting the RSV F protein, with a focus on combating respiratory syncytial virus infections. With its current highest phase being Phase 1 globally and preclinical in China, the vaccine shows potential in addressing the significant health burden caused by RSV infections.