Clover Shares Initial Positive Phase I Results for SCB-1019, a Bivalent RSV Vaccine in Seniors
Clover Biopharmaceuticals, Ltd., an internationally operating biotechnology firm focused on leveraging groundbreaking vaccines for global health improvement, has shared favorable initial immunogenicity and safety results from the elder and senior groups in its Phase Ⅰ trial. This trial assesses SCB-1019, Clover’s bivalent RSV prefusion-stabilized F (PreF)-Trimer subunit vaccine candidate, developed using the company’s proprietary Trimer-Tag vaccine technology platform.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"SCB-1019, the first RSV PreF vaccine candidate from China to advance to clinical trials and generate clinical data, has shown promising Phase I results in older adults," stated Joshua Liang, Chief Executive Officer & Board Director of Clover. "The vaccine elicited broad and significant neutralizing antibody responses against both RSV-A and RSV-B, coupled with a favorable safety profile. We are eager to see the complete Phase I clinical results by the end of 2024 to facilitate further development and solidify our unique standing in global markets."
Neutralizing antibody responses against both RSV-A and RSV-B from the SCB-1019 trial are comparable or potentially superior to other protein subunit RSV PreF vaccines. This supports Clover's bivalent approach, especially given that previous monovalent RSV-A vaccines have shown lower immune responses or reduced efficacy against RSV-B.
Additionally, the results confirm that Clover's PreF antigens in SCB-1019 retain their stabilized prefusion and trimeric forms. Exploratory immunogenicity data also show significant increases in Site Ø and Site V neutralizing antibody-competitive titers.
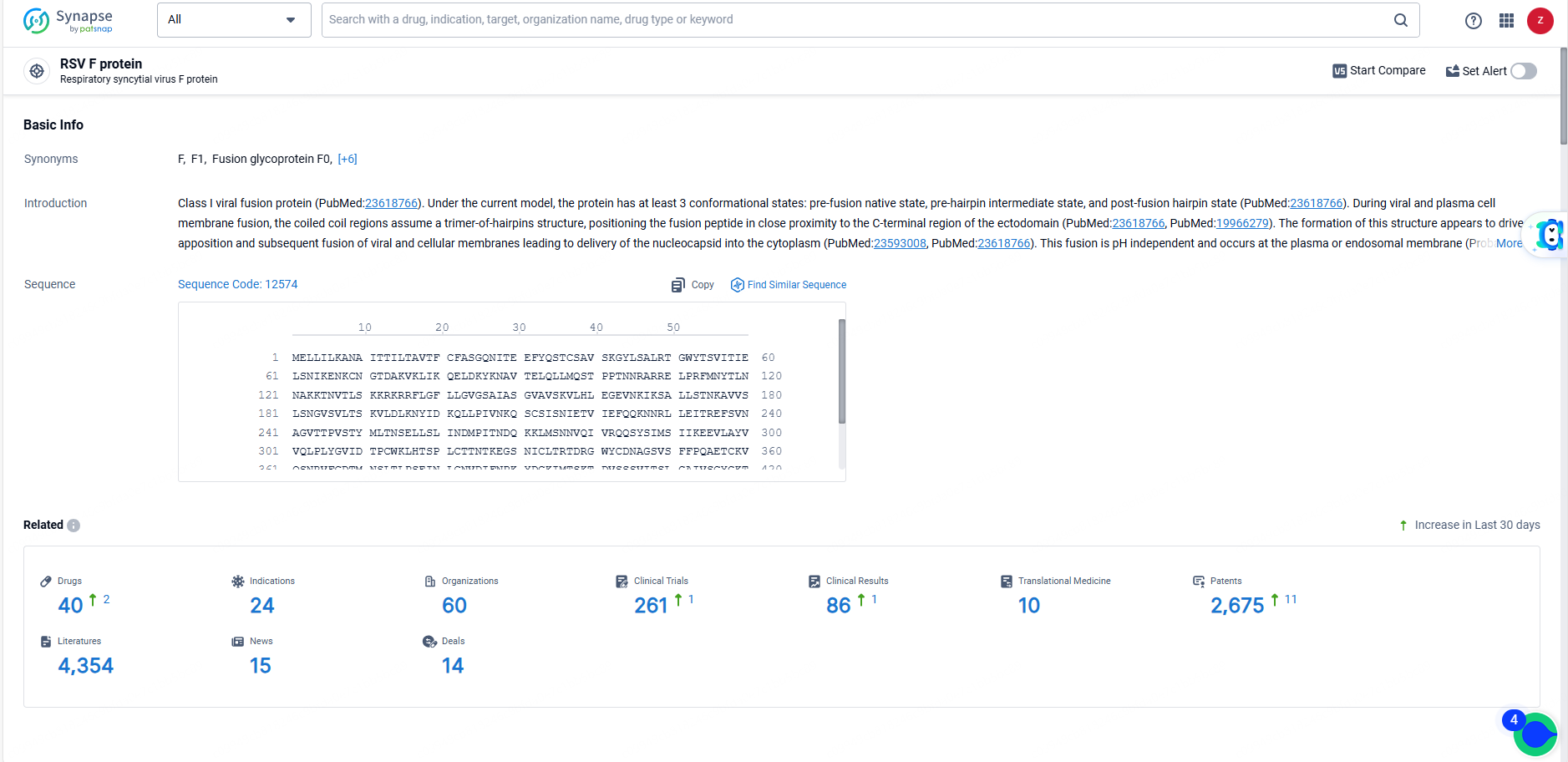
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 40 investigational drugs for the RSV F protein targets, including 24 indications, 60 R&D institutions involved, with related clinical trials reaching 261, and as many as 2675 patents.
SCB-1019 is a vaccine targeting the RSV F protein, with a focus on treating respiratory syncytial virus infections. the RSV F antigen vaccine developed by Clover Biopharmaceuticals is a promising candidate in the field of biomedicine, with the potential to make a significant impact on the prevention and treatment of RSV infections. Its progression to Phase 1 in global development reflects the ongoing efforts to advance the vaccine towards potential regulatory approval and eventual commercialization.