Is Brolucizumab-dbll approved by the FDA?
Yes, brolucizumab-dbll, marketed under the brand name Beovu, is FDA approved. The FDA approved Beovu on October 7, 2019, for the treatment of neovascular (wet) age-related macular degeneration (AMD). This approval marked a significant advancement in the management of AMD, providing a new option for patients suffering from this vision-impairing condition.
What is Brolucizumab-dbll?
Brolucizumab-dbll is an anti-angiogenic ophthalmic agent used primarily for treating neovascular (wet) age-related macular degeneration (AMD) and diabetic macular edema (DME). These conditions are characterized by abnormal blood vessel growth in the eye, leading to vision impairment and, potentially, blindness. Brolucizumab-dbll helps by reducing the amount of blood flow to these abnormal vessels, thus mitigating the damage to the retina.
How to Use Brolucizumab-dbll
Brolucizumab-dbll is administered as an injection into the eye by an ophthalmologist. The dosing schedule varies based on the condition being treated:
- For Neovascular AMD: The typical regimen involves an injection every month for the first three doses, followed by injections every 8 to 12 weeks.
- For Diabetic Macular Edema (DME): The regimen starts with an injection every six weeks for the first five doses, followed by injections every 8 to 12 weeks.
Precautions and Considerations
Before Using Brolucizumab-dbll:
- Discuss any allergies or medical conditions with your doctor, particularly if you have a history of blood clots, detached retina, endophthalmitis, glaucoma, heart attack, stroke, or active eye infections.
- The safety and efficacy of brolucizumab-dbll in pediatric patients have not been established.
- For pregnant or breastfeeding women, the potential risks and benefits should be carefully weighed.
Interactions:
- Inform your healthcare provider about any other medications you are taking, including prescription, over-the-counter drugs, vitamins, and herbal supplements.
Potential Side Effects
Common Side Effects:
- Blurred vision
- Eye redness
- Decreased vision
- Blindness
- Bloodshot eyes
Less Common Side Effects:
- Burning, dry, or itching eyes
- Excessive tearing or discharge
- Headache
- Painful eye irritation
- Seeing flashes of light or floating spots
Serious Side Effects:
- Eye infections
- Increased eye pressure
- Retinal detachment
- Vasculitis or vascular blockage
If you experience any severe side effects or changes in vision, contact your eye doctor immediately.
Conclusion
Brolucizumab-dbll (Beovu) was approved by the FDA on October 7, 2019, for the treatment of neovascular AMD and diabetic macular edema. Administered via injection by an ophthalmologist, it offers a new treatment option for patients with these serious eye conditions. As with any medication, it is crucial to follow your healthcare provider's instructions and attend all follow-up appointments to monitor for any adverse effects. Always discuss your full medical history and any medications you are taking with your healthcare provider to ensure the safe and effective use of brolucizumab-dbll.
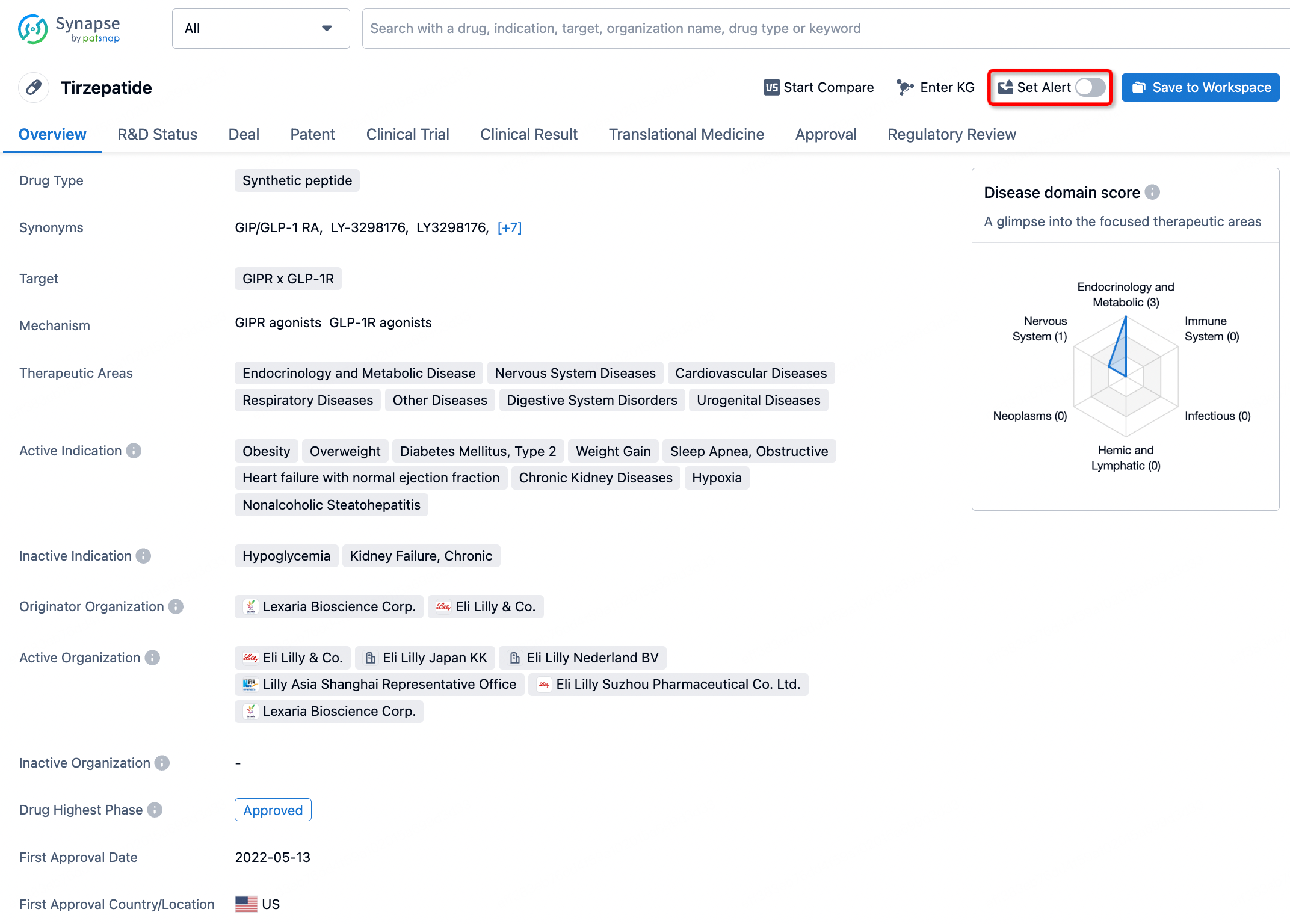
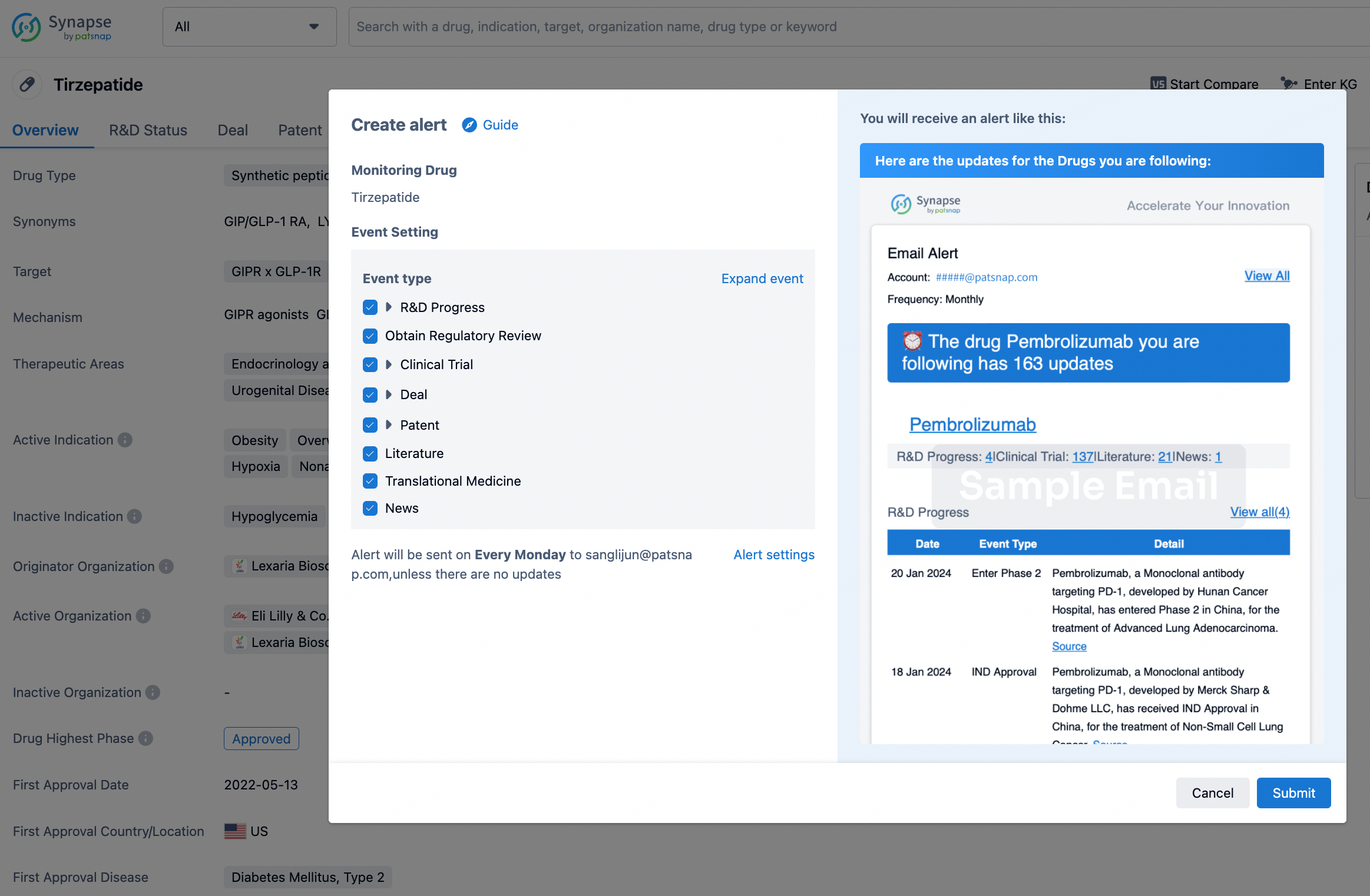
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!