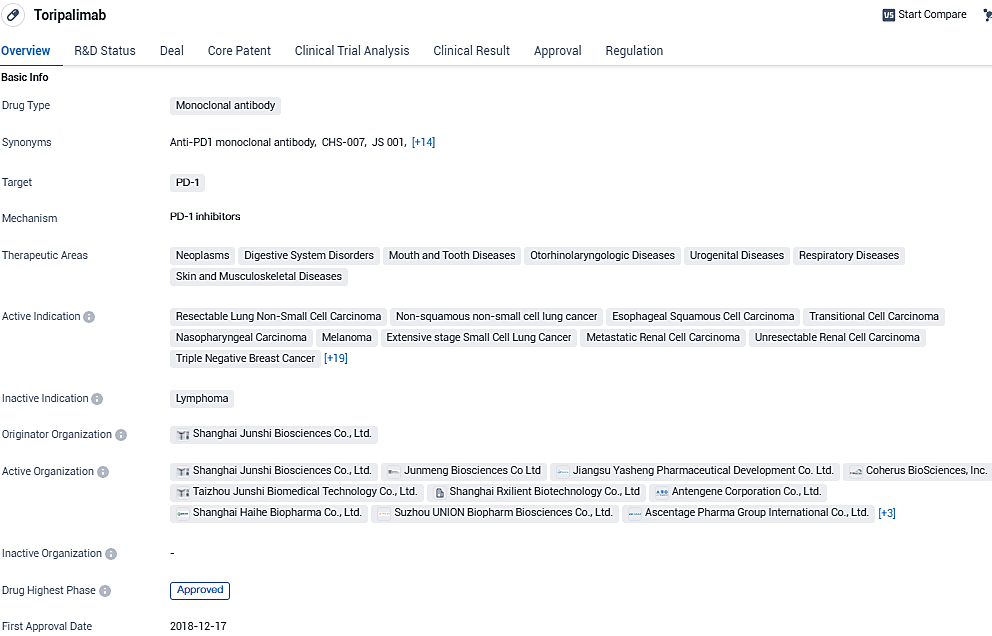
Coherus Reveals the Introduction of LOQTORZI™ in the United States Market
Coherus BioSciences, Inc. has recently declared that their product, LOQTORZI™ (toripalimab-tpzi), can now be acquired from certain specialty distribution channels within the U.S. market. This medication is prescribed for use together with cisplatin and gemcitabine as an initial therapeutic option for adult patients who are dealing with metastatic or locally advanced nasopharyngeal carcinoma that has recurred. Additionally, LOQTORZI is approved to be administered as a single-agent therapy for adult patients grappling with nasopharyngeal carcinoma that is recurrent, cannot be surgically removed, or is metastatic, specifically in cases where the disease has advanced despite prior treatment with platinum-based chemotherapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
LOQTORZI is an innovative biotherapeutic designed to inhibit the programmed death receptor-1 (PD-1), particularly its ligands PD-L1 and PD-L2. It binds with exceptional affinity to a distinct site on the PD-1 receptor, potentiating the immune response to identify and eliminate cancer cells.
John Hopper, who is actively involved with initiatives such as the Patient Activation Group and NORD Rare Cancer Coalition, in addition to his leadership role at the rare cancer foundation SARC, remarked on the challenging landscape faced by individuals with uncommon malignancies like nasopharyngeal carcinoma (NPC). He stressed that the introduction of LOQTORZI as a treatment for advanced NPC offers a substantial advancement in patient care, bringing a renewed sense of optimism to those advocating for more diverse treatment avenues for rare cancer instances.
In the United States, NPC impacts roughly 2,000 patients each year. Historically, these patients have relied on chemotherapy as their primary regimen. However, after considering data from the phase 3 JUPITER-02 study and the phase 2 POLARIS-02 study, the NCCN Committee in December recommended LOQTORZI, combined with gemcitabine and cisplatin, as an optimal category 1 treatment.
Coherus aims to redefine the current approach to treating relapsed/metastatic NPC, explains Paul Reider, the company's Chief Commercial Officer. LOQTORZI is a pioneer in this respect, with no previously FDA-approved alternatives for patients. Efficacy markers such as progression-free survival (PFS) and overall survival (OS) have highlighted its potential for improving outcomes for patients with R/M NPC, offering a beacon of hope for increased longevity.
Denny Lanfear, Coherus’ Chairman, and CEO proclaimed that the unveiling of LOQTORZI marks a pivotal point for the company, as it steps into the limelight of the immuno-oncology market. Coherus foresees that LOQTORZI will be instrumental among a new wave of cancer immunotherapies that aim to enhance life expectancy in patients faced with various cancer types, bolstered by the innovative developments within its infrastructure and cooperative ventures.
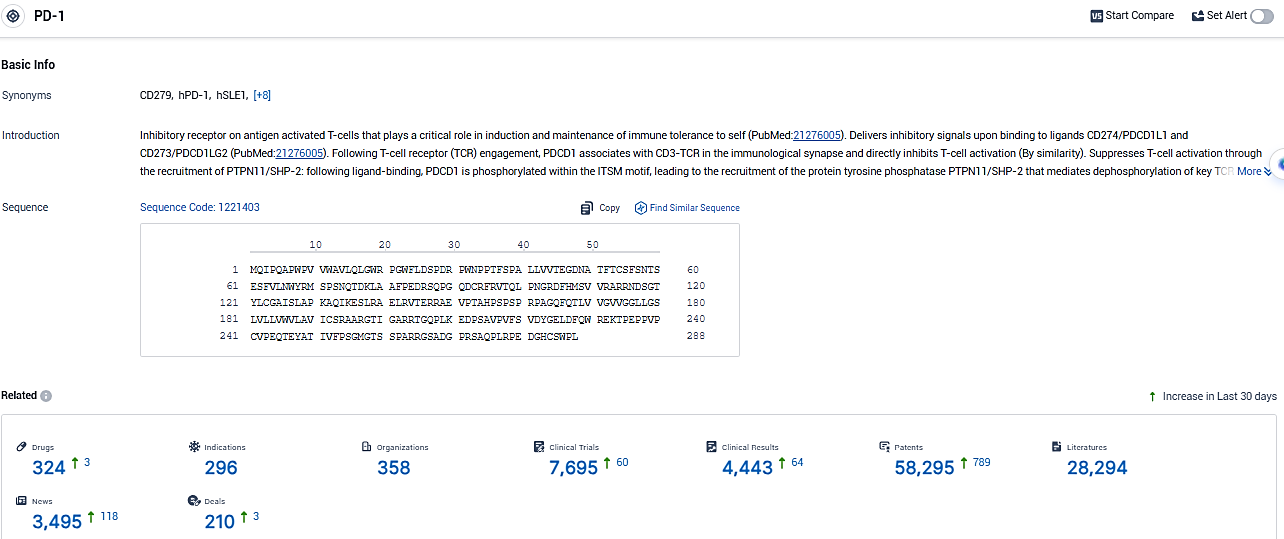
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 6, 2024, there are 324 investigational drugs for the PD-1 target, including 296 indications, 358 R&D institutions involved, with related clinical trials reaching 7695, and as many as 58295 patents.
LOQTORZI is a next generation anti-PD-1 monoclonal antibody that blocks PD-L1 binding to the PD-1 receptor at a unique site with high affinity and activates antitumor immunity demonstrating improvement in the overall survival of cancer patients in several tumor types. LOQTORZI is commercially available for purchase through select specialty distributors including Cencora, Cardinal and McKesson.