Combating Obesity with GLP-1RAs: From Semaglutide to Small-Molecule Agonists and the Role of Patent Analytics in Drug Development
Amid the growing global obesity crisis, the number of obese children and adults worldwide reached 157 million and 764 million, respectively, in 2020. By 2030, it is projected that the global obese population will soar to a staggering 1 billion. Faced with this global health challenge, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have garnered significant attention for their dual effects in glucose control and weight reduction.
Novo Nordisk’s semaglutide, as a representative of second-generation GLP-1RAs, has become a market sensation since gaining approval for obesity indications in 2021, propelling Novo Nordisk to become one of the highest-valued publicly traded companies in Europe. The success of semaglutide lies not only in its remarkable weight-loss efficacy but also in the hope it has brought to obesity treatment. However, competition in the weight-loss market remains fierce. Eli Lilly's tirzepatide is a notable contender. In November 2023, tirzepatide received FDA approval for the long-term weight management of adults with obesity or overweight. According to data released by Eli Lilly, tirzepatide achieved 1.47 times the relative weight reduction compared to semaglutide, outperforming semaglutide on the primary endpoint as well as five key secondary endpoints.
In this race to combat obesity, Gan & Lee Pharmaceuticals has also joined the fray. On December 11, 2024, the company initiated its first Phase III trial, GZR18-BWM-301, for its long-acting GLP-1 receptor agonist GZR18 injection. Phase IIb clinical data in Chinese adults with obesity/overweight demonstrated that after 30 weeks of treatment, the mean percentage change in body weight from baseline ranged from -11.15% to -17.78%, compared to -0.99% in the placebo group, highlighting the potential of GZR18 injection in weight loss.
All the aforementioned therapies are peptide-based drugs, but the competition in the small-molecule GLP-1 receptor agonist field is equally intense. Leading multinational corporations (MNCs) such as Eli Lilly, Pfizer, Roche, AstraZeneca, and Gilead have all entered the small-molecule GLP-1RA race. On August 21, 2024, Gilead registered a Phase I clinical trial for its small-molecule GLP-1 receptor agonist GS-4571 for diabetes and obesity treatment on ClinicalTrials.gov. Merely half a month later, biopharmaceutical company Terns Pharmaceuticals, Inc. announced the Phase I trial results of its oral small-molecule GLP-1RA TERN-601.
Meanwhile, Eli Lilly is leading the small-molecule GLP-1RA race with its candidate orforglipron, which has already advanced to Phase III clinical trials.
Next, let’s take Eli Lilly’s Orforglipron as an example to explore its patent landscape using Patsnap Chemical. We will examine Eli Lilly’s patent layout for Orforglipron, identify which pharmaceutical companies are fast-followers of Orforglipron, and analyze the patent landscape in the small-molecule GLP-1 receptor agonist field.
First, we log in to Patsnap Chemical. Select the Structure Search option and input Orforglipron’s commonly used identifiers (e.g., CAS number, common substance name, molecular formula, SMILES file, etc.). Alternatively, you can directly edit the structure in the structure editor or upload a structure file (MOL format) created using ChemDraw. By performing a similarity search (with the Tanimoto coefficient set to 0.7), we can retrieve patent applications for compounds structurally similar to Orforglipron.
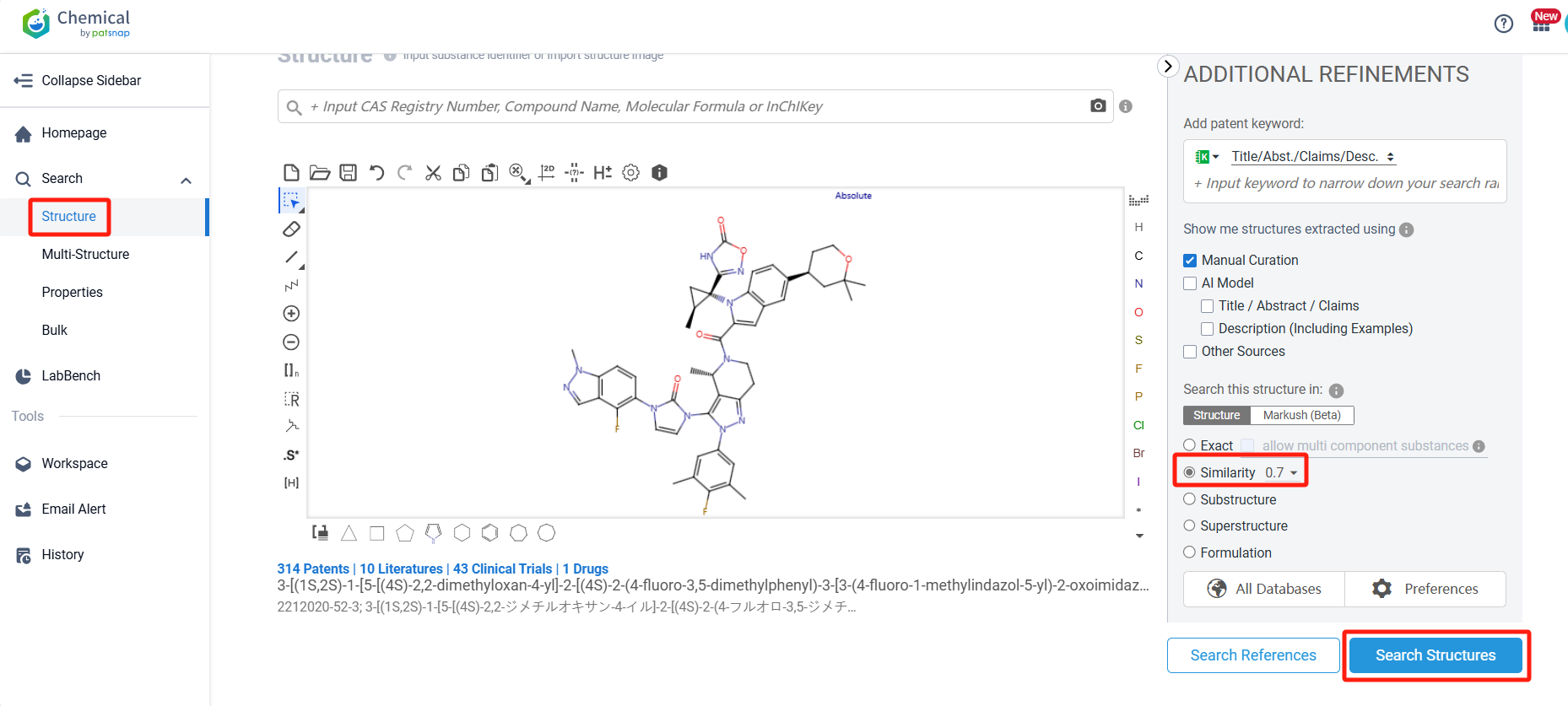
After initiating the compound search, we can access information related to Orforglipron, including compounds, patents, and literature. Here, we find that there are 720 patents associated with Orforglipron. To simplify the review process, we can click on the "View in Analytics" link to access the related patent data via Patsnap Analytics.
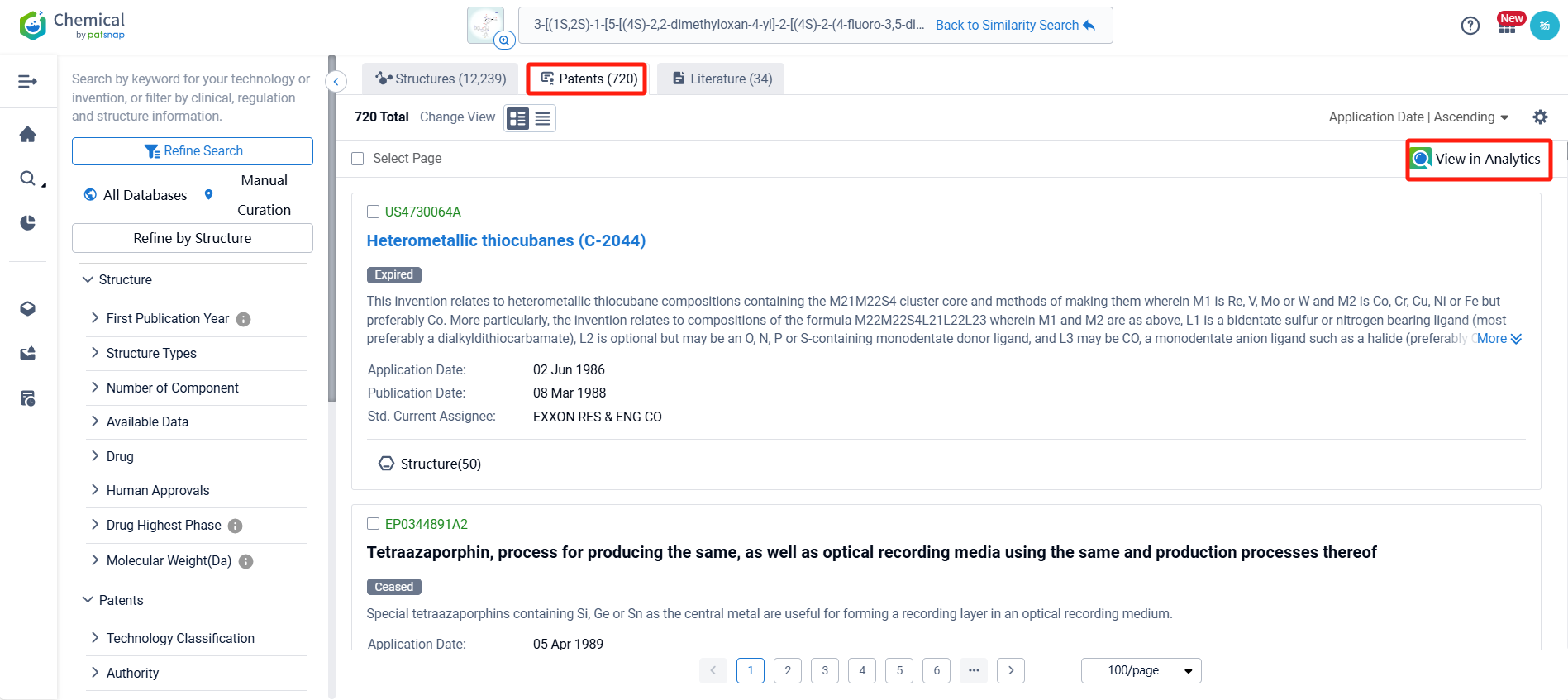
After linking to Patsnap Analytics, we refined the retrieved patents for easier analysis by applying filters for application fields and technical themes. Specifically, we selected "Pharmaceutical Sciences" under application fields and "Chemistry" and "Medicine" under technical themes. After applying these filters, the number of relevant patents was reduced to 103.
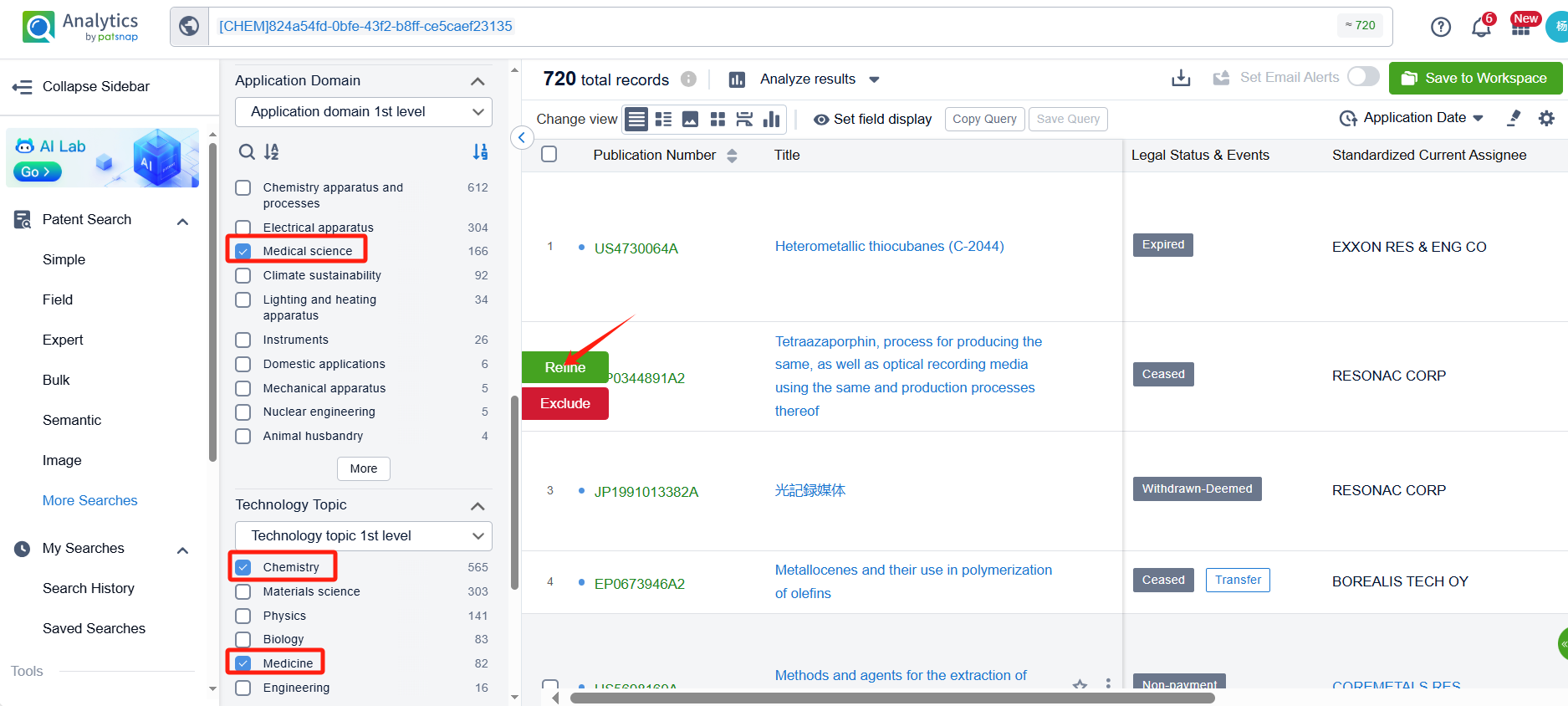
Sorting by filing date, we found that Orforglipron was first disclosed in the PCT international application WO2018056453A1 by Chugai Pharmaceutical Co., Ltd. (filing date: September 26, 2017; publication date: March 29, 2018). This patent has been granted in the United States, Europe, China, and Japan.
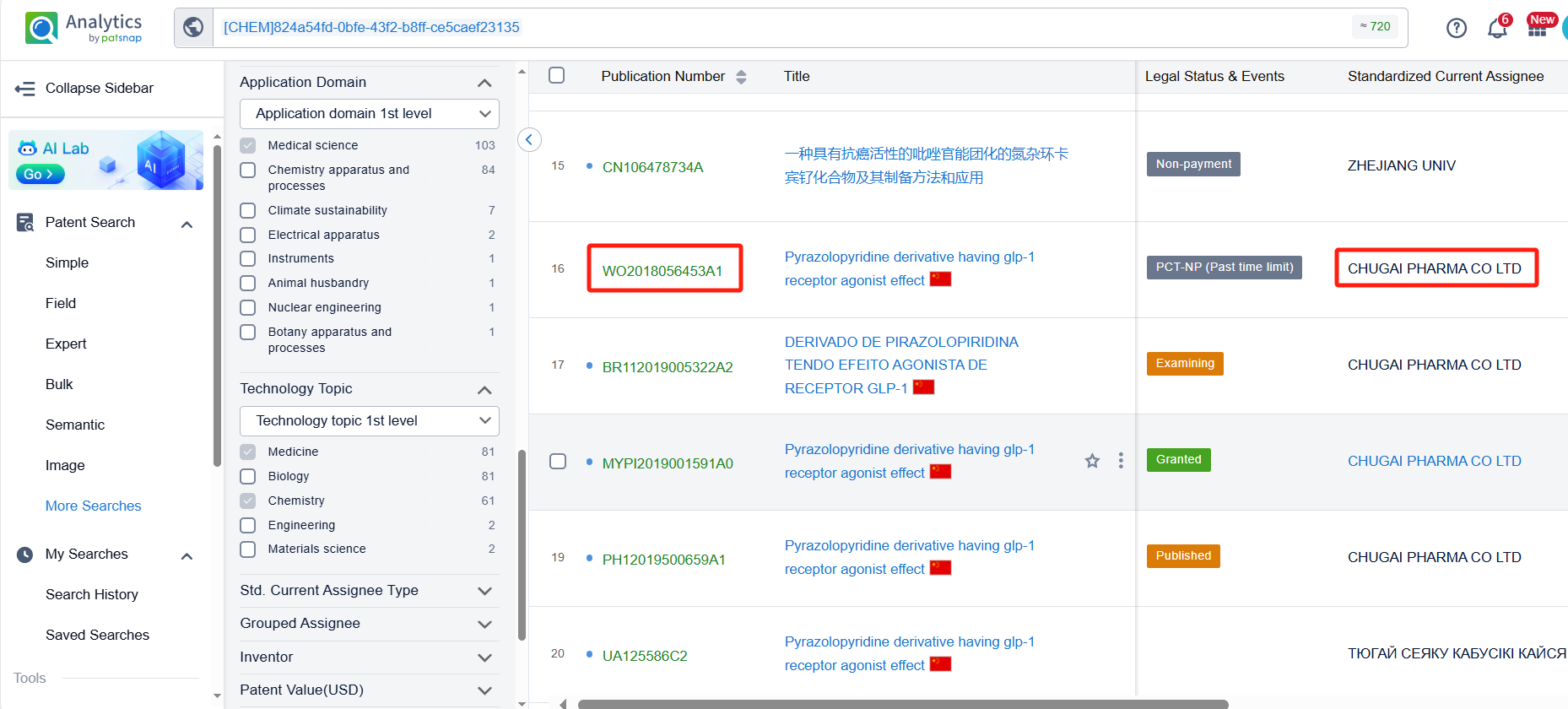
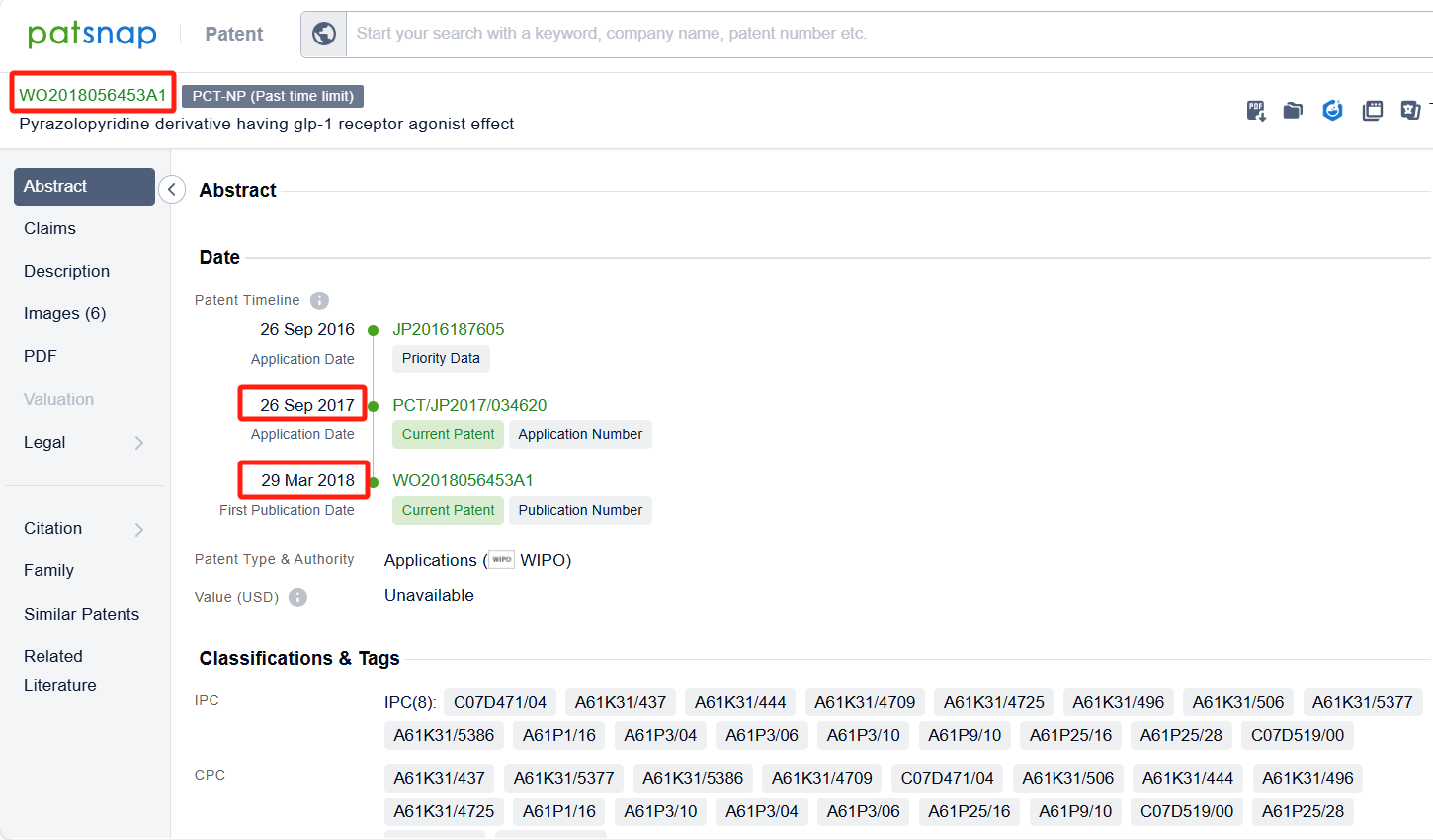
To facilitate the review of the patent layouts for the research companies (Chugai Pharmaceutical Co., Ltd. and Eli Lilly & Co.) related to Orforglipron, we applied a filter by research companies, narrowing the results to 9 patents. Analyzing these patents revealed that Chugai Pharmaceutical Co., Ltd., aside from its core patent WO2018056453A1 and its family members, has no other patents related to Orforglipron.
In contrast, Eli Lilly has filed additional patents for Orforglipron-related derivatives, such as patents for tablets (WO2023220112A1), capsules (WO2023220109A1), and dosage regimens for treating type 2 diabetes (T2D) and obesity (WO2024129676A1).
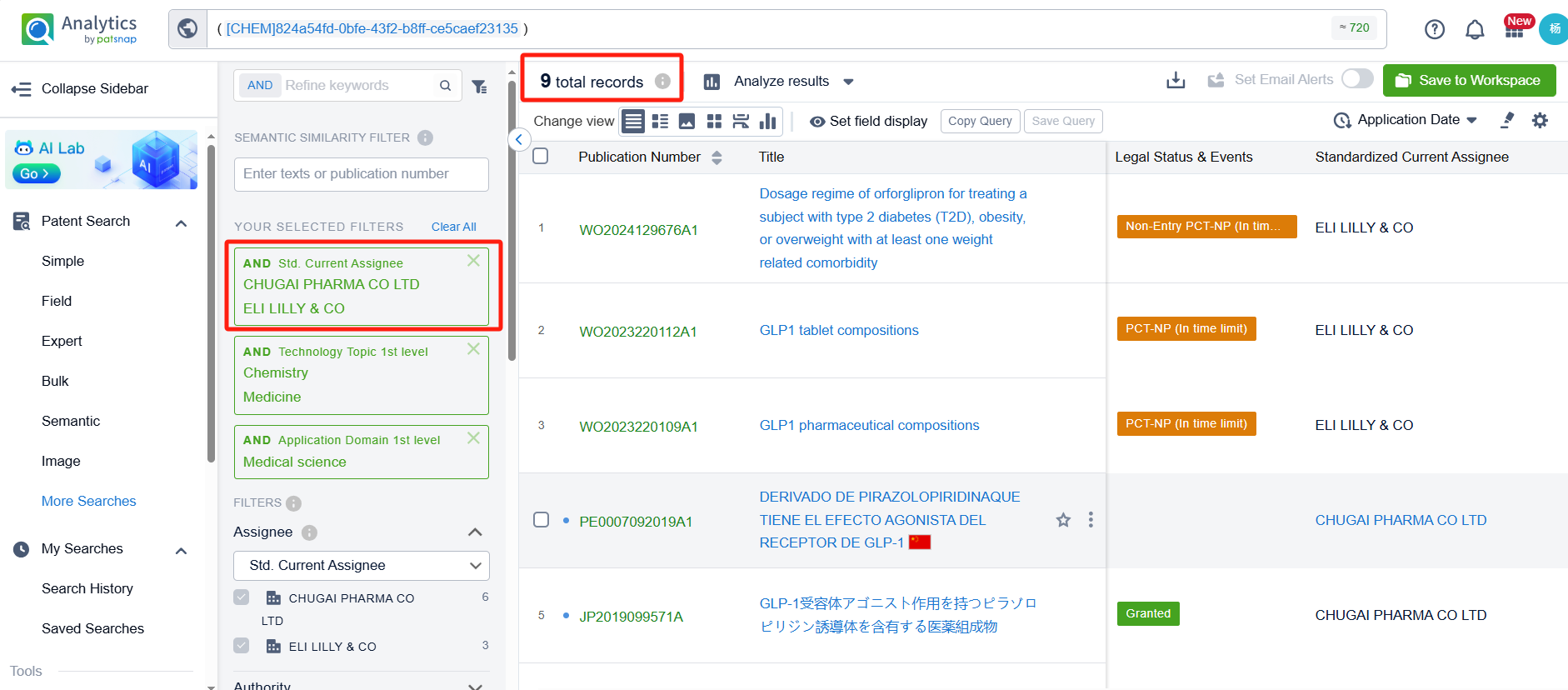
To identify which pharmaceutical companies are fast-followers of Orforglipron, we applied a filter for research companies. After refinement, 34 patents remained. By clicking on the publication numbers of these patents, we could directly access their detailed information.
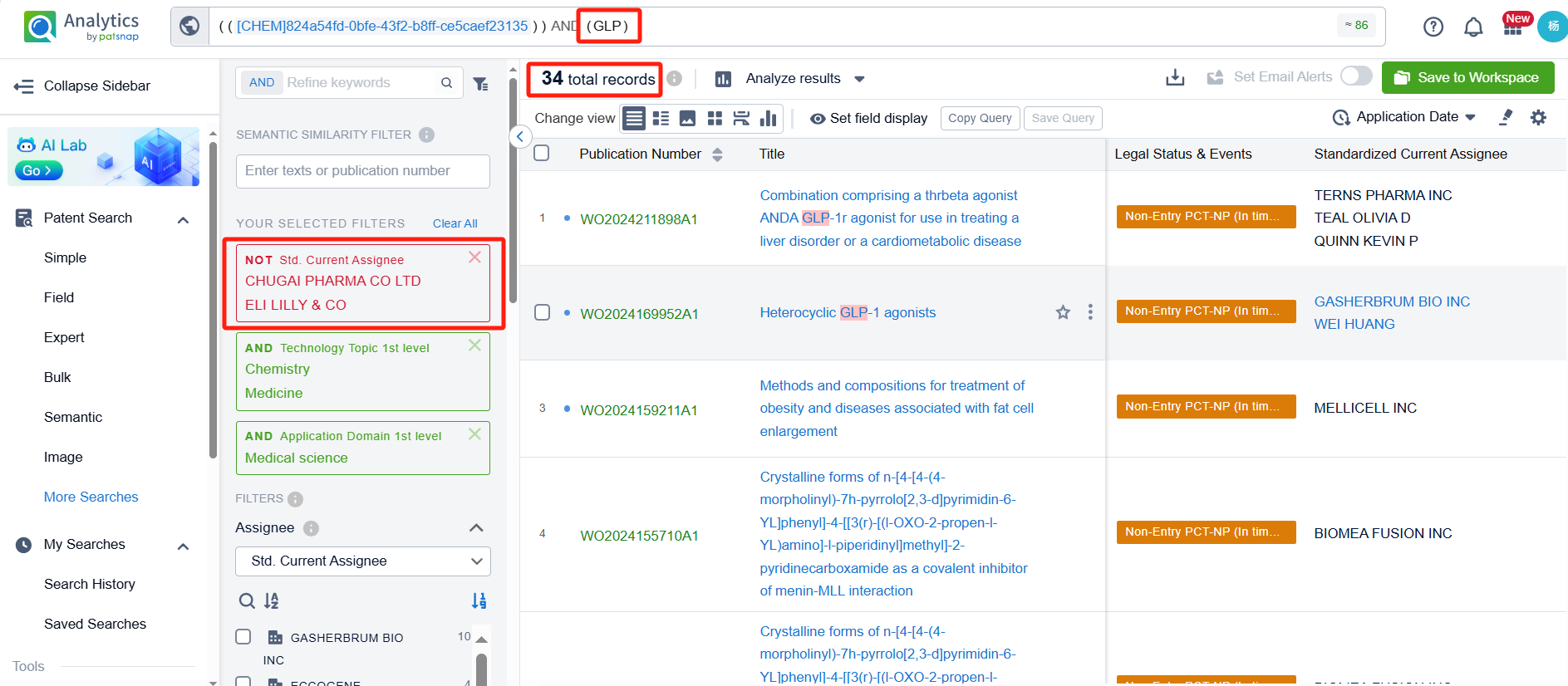
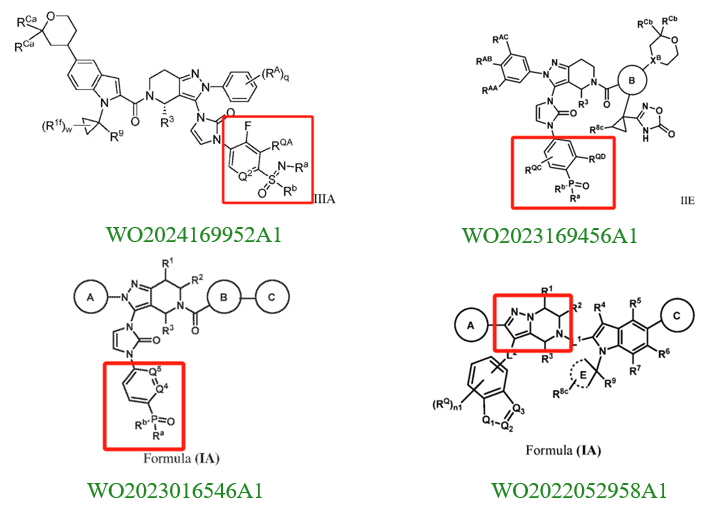
Upon reviewing these patents, we found that companies involved in patent applications for structures similar to Orforglipron include Gasherbrum Bio, Inc., Fochon Biosciences Co., Ltd., Beijing Shenogen Pharma Group Ltd., and Eccogene (Shanghai) Co., Ltd. Notably, Gasherbrum Bio, Inc. has filed six patent applications related to structures similar to Orforglipron. Below, we provide a detailed analysis of the patents filed by each company.
For instance, the patent CN117069743A (filed on May 10, 2022, and published on November 17, 2023) by Beijing Shenogen Pharma Group Ltd. primarily focuses on modifications to the indole ring of Orforglipron. The patent includes only two specific compounds in its examples, with the primary difference being the substitution of a carbon atom on the indole ring with a nitrogen atom. However, Orforglipron's core patent (US20190225604A1) also discloses the possibility of replacing the carbon atom on the indole ring with a nitrogen atom. While experimental data from the newer patent suggests improved GLP-1 receptor agonist activity compared to Orforglipron, the extent of the improvement might not be sufficient to establish inventive step for these compounds.
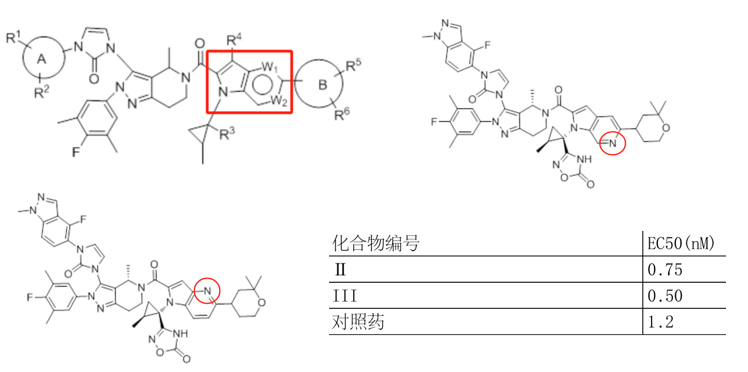
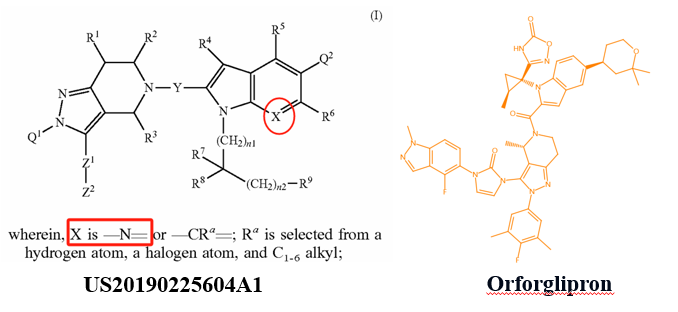
Unlike the compound modifications in Beijing Shenogen Pharma Group's patent, which are limited to the indole ring, Fochon Biosciences’ patent WO2024153070A1 (filed on January 16, 2024, and published on July 25, 2024) encompasses broader modification sites. Based on the provided examples, the structural changes compared to Orforglipron primarily involve both the indole ring and the oxadiazole ring in the side chain (W substituent). Experimental data indicate that the compounds in this patent exhibit strong GLP-1 receptor agonist activity. The combination of these two aspects is likely critical in determining both the patent's approval status and the scope of its claims.
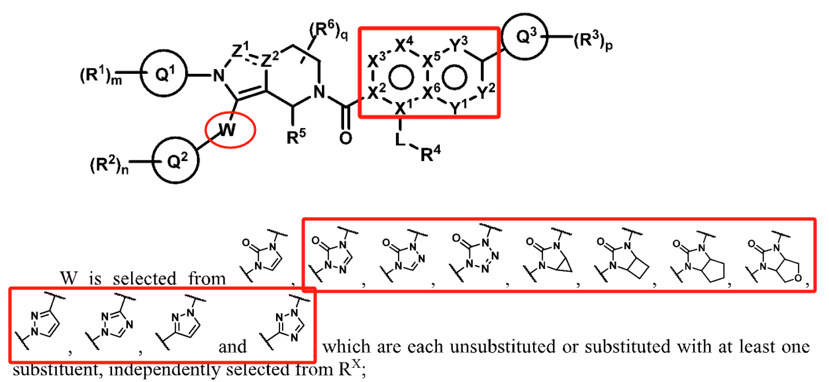
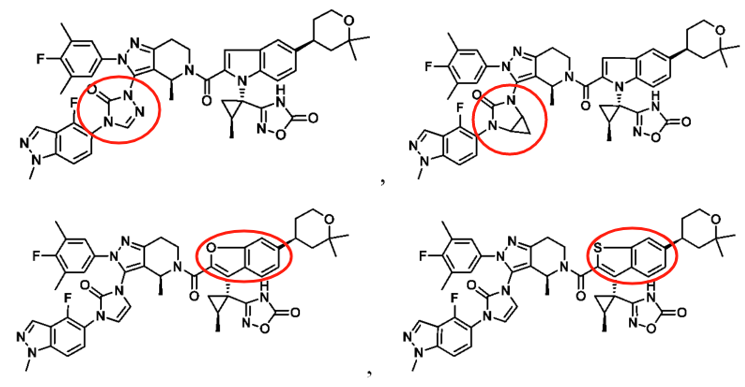
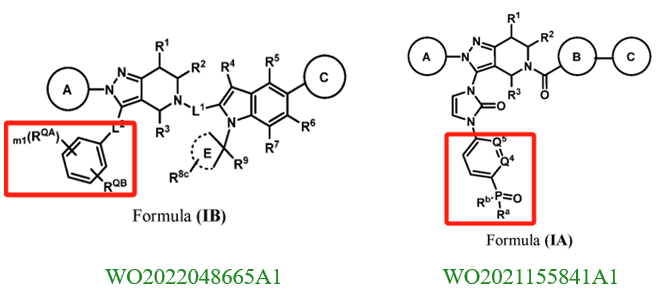
Eccogene's patent WO2022017338A1 (filed on July 20, 2021, and published on January 27, 2022) also focuses on modifications to the indole ring. However, its primary innovation lies in relocating the nitrogen atom from the indole ring to a bridge structure of the indole system. Experimental results show that these compounds have strong GLP-1 receptor agonist activity and favorable pharmacokinetic properties. This patent has already been granted in the US and Japan. From the granted claims, it is evident that further specific limitations were applied to ring A.
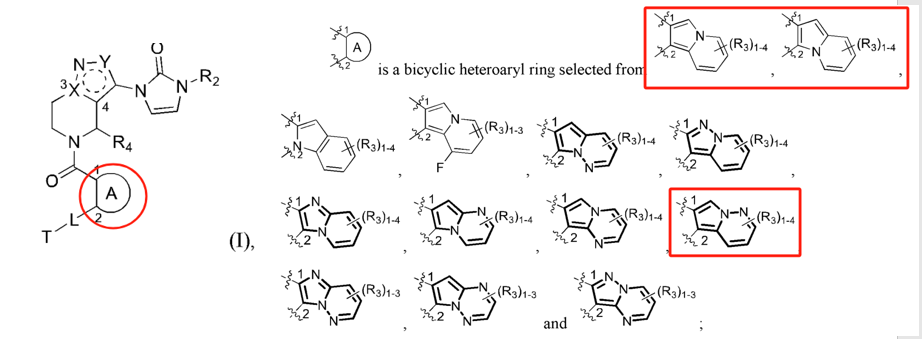
A review of Gasherbrum Bio's six related patents reveals that the patent WO2022052958A1 (filed on September 8, 2021, and published on March 17, 2022) focuses on modifications to the pyrazolopyridine ring. Its primary innovation is relocating the nitrogen atom on the pyrazole ring to the bridge structure of the pyrazolopyridine system, a modification method similar to Eccogene’s WO2022017338A1. The remaining five patent applications focus on modifications to the indazole substituent in the side chain. These involve replacing the indazole with a phenyl group and introducing hydrophilic substituents (e.g., phosphoryl or sulfonyl groups) to improve pharmacokinetic properties. Among these, the PCT application WO2021155841A1 (filed on February 5, 2021, and published on August 12, 2021) has been granted in the US, China, and Europe.
From the patent applications of various companies, it is evident that the fast-followers of Orforglipron are primarily Chinese companies, reflecting the enthusiasm of Chinese pharmaceutical firms for innovative drug development. It has been predicted that the global anti-obesity drug market could reach a total size of $100 billion by 2030. For many pharmaceutical companies, this represents an undeniably attractive market. In the future, it is inevitable that more companies will enter the small molecule GLP-1 receptor (GLP-1R) agonist field, leading to the emergence of more high-potential GLP-1R agonists that offer additional treatment options for patients with obesity.
The immense market potential of small molecule GLP-1R agonists, coupled with intense R&D competition, is likely to result in even more fast-followers. This underscores the critical importance of conducting Freedom to Operate (FTO) analyses. Patsnap Chemical, with its powerful search capabilities and rich data resources, seamlessly connects chemical structures with drug information, including patents, literature, and clinical trials. This bidirectional linkage facilitates chemical structure FTO searches, R&D patent investigations, and decision-making in new drug development and intellectual property protection. By leveraging such tools, we can make more informed decisions during the drug discovery process and ensure robust IP strategies.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.




