Comprehensive understanding of Proton Pump Inhibitors
Proton pumps are enzymes found in the stomach lining that play a crucial role in the production of gastric acid. These pumps are responsible for the secretion of hydrogen ions into the stomach, which helps in the digestion of food. By regulating the acidity levels in the stomach, proton pumps aid in the breakdown of proteins and the absorption of essential nutrients. However, excessive acid production can lead to conditions like gastroesophageal reflux disease (GERD) and peptic ulcers. Therefore, pharmaceutical companies have developed proton pump inhibitors (PPIs) to selectively inhibit these pumps, reducing acid secretion and providing relief from acid-related disorders.
PPIs act on H+-K+-ATPase, effectively inhibiting both basal and various stimuli-induced gastric acid secretion. These medicines have fast onset, powerful and lasting acid-suppressing effects, and offer good safety and tolerance. They are the first choice of drugs for the clinical treatment and prevention of acid-related diseases. PPIs are widely used in the treatment of acute and chronic digestive system acid-related diseases, including gastroesophageal reflux disease, peptic ulcer, upper gastrointestinal bleeding, and other related diseases. They are also applied in eradicating Helicobacter pylori (H. pylori) infection, and in the prevention and treatment of stress-related gastromucosal lesions.
With the long-term and extensive use of PPIs, combined with an ever-expanding user population, the issue of PPI overuse (super-indication, overdose, and over-treatment duration) has become increasingly prominent. Among the global patients receiving PPI treatment, 25% to 70% of prescriptions are improperly managed, leading to an unnecessary annual cost of up to 2 billion pounds due to PPI overuse. This significantly increases the economic burden of patients and health insurance departments.
Proton pump Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 3 Sep 2023, there are a total of 82 Proton pump drugs worldwide, from 120 organizations, covering 65 indications, and conducting 2109 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.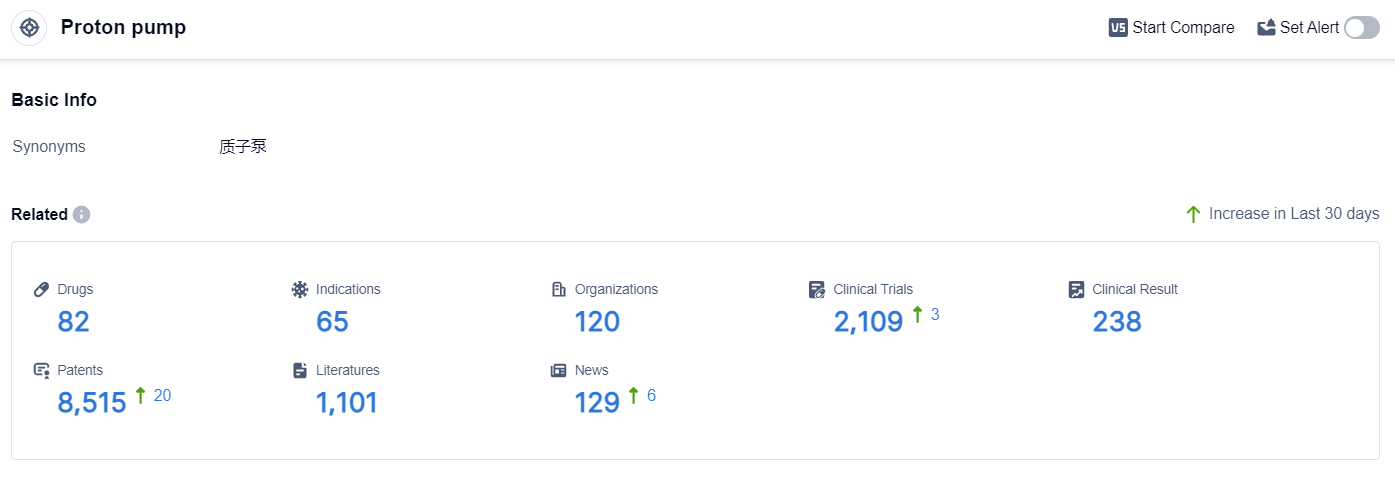
The analysis of the current competitive landscape of the target Proton pump reveals that Takeda Pharmaceutical Co., Ltd., AstraZeneca PLC, and Eisai Co., Ltd. are the companies growing fastest in this field. These companies have made significant progress in the research and development of drugs targeting the Proton pump. The indications with the highest number of approved drugs include Duodenal Ulcer, Stomach Ulcer, and Gastroesophageal Reflux. The presence of a large number of small molecule drugs indicates intense competition around the innovative drug. China, Japan, and the United States are the countries/locations that are developing fastest under the current target Proton pump, with China showing significant progress in drug development. The future development of the target Proton pump is expected to be driven by the continued research and development efforts of these companies and the exploration of new indications and drug types.
Classification of Proton Pump Inhibitors
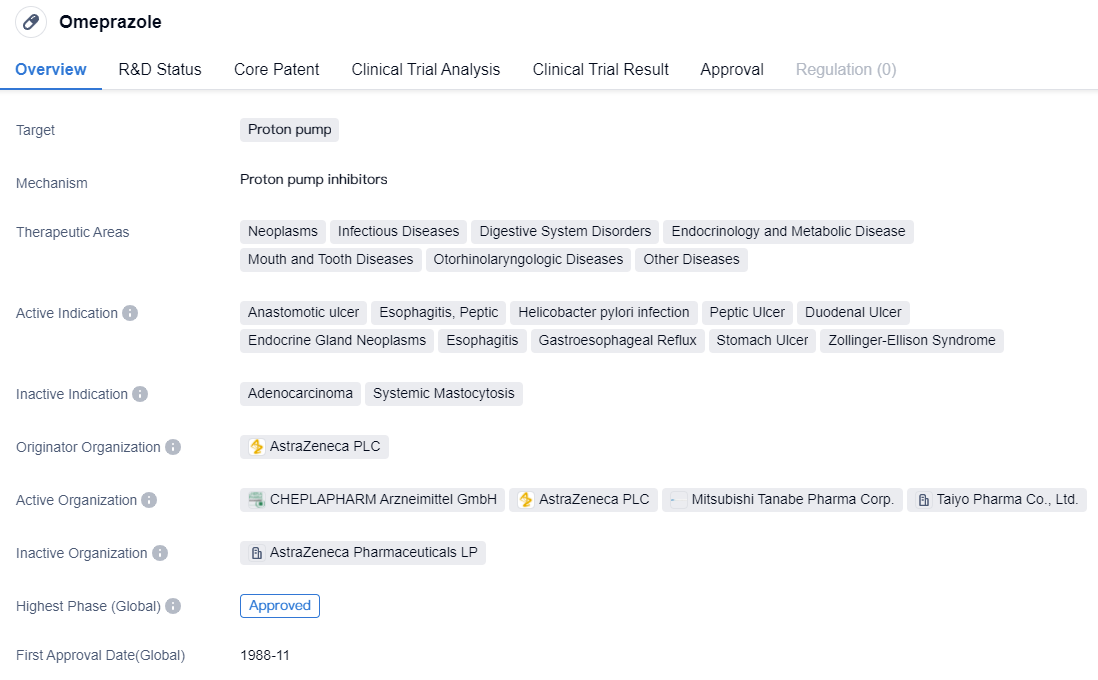
After the market introduction of Omeprazole in 1988, a series of proton pump inhibitors have sequentially appeared. Based on whether they can reversibly inhibit H+-K+-ATPase (proton pump), they are divided into irreversible proton pump inhibitors and reversible proton pump inhibitors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Irreversible proton pump inhibitors, mainly metabolized by isoenzymes CYP2C19 and CYP3A4 in the liver CYP450 enzyme system, can irreversibly inactivate H+-K+-ATPase. Due to the different genotypes and activity levels between individuals, there is a significant individual variation in the therapeutic effects of some irreversible proton pump inhibitors. They can further be classified into CYP2C19-dependent proton pump inhibitors and CYP2C19-independent proton pump inhibitors.
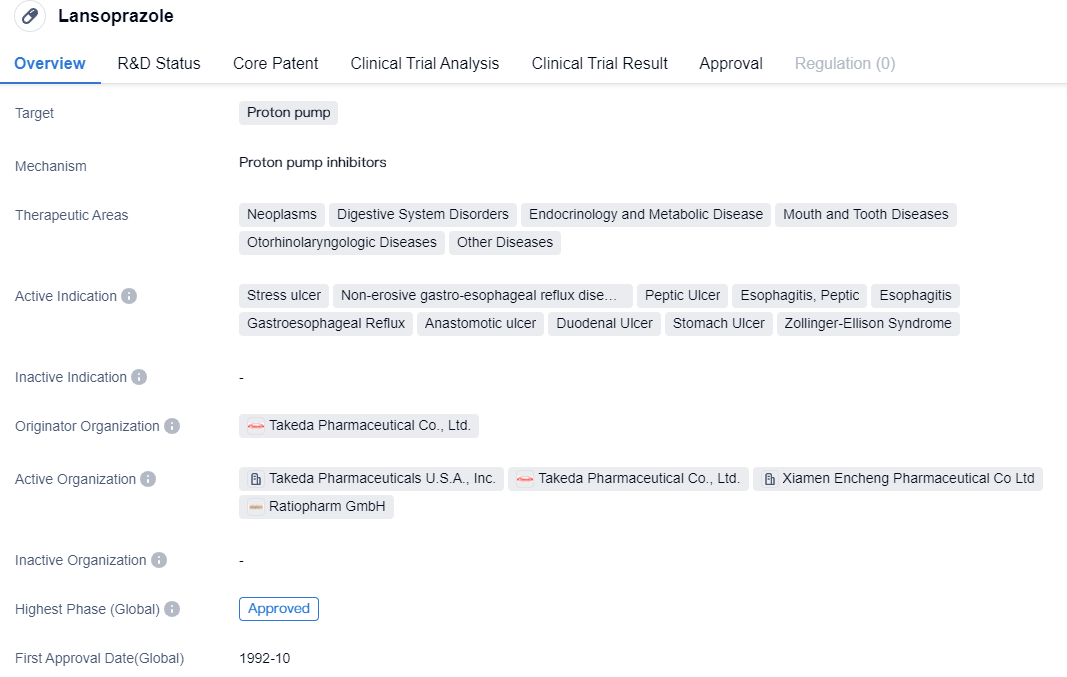
CYP2C19-dependent proton pump inhibitors include drug representatives such as Omeprazole, Lansoprazole, etc., which belong to the first generation of proton pump inhibitor drugs. These drugs suffer from issues such as low bioavailability, short half-life, large individual differences, drug interactions, unstable acid suppression, slow onset, nocturnal acid breakthrough, rebound of gastric acid secretion, etc. It is suggested that medical institutions with the necessary resources should carry out gene typing tests, monitor blood drug concentrations, make reasonable drug selections to improve efficacy and reduce adverse reactions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
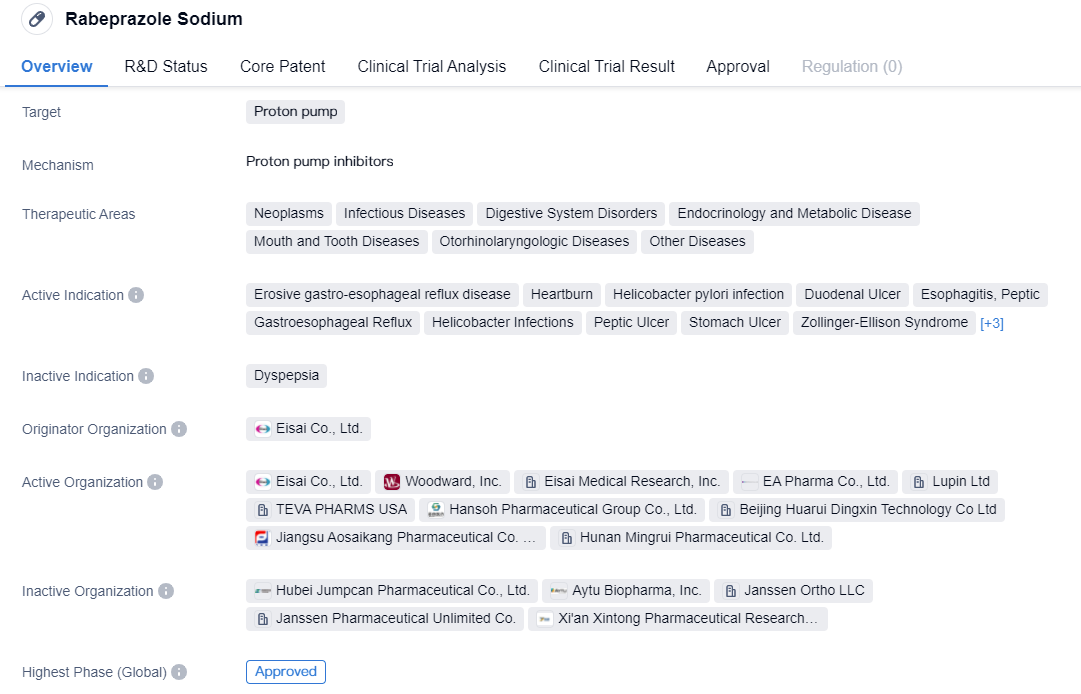
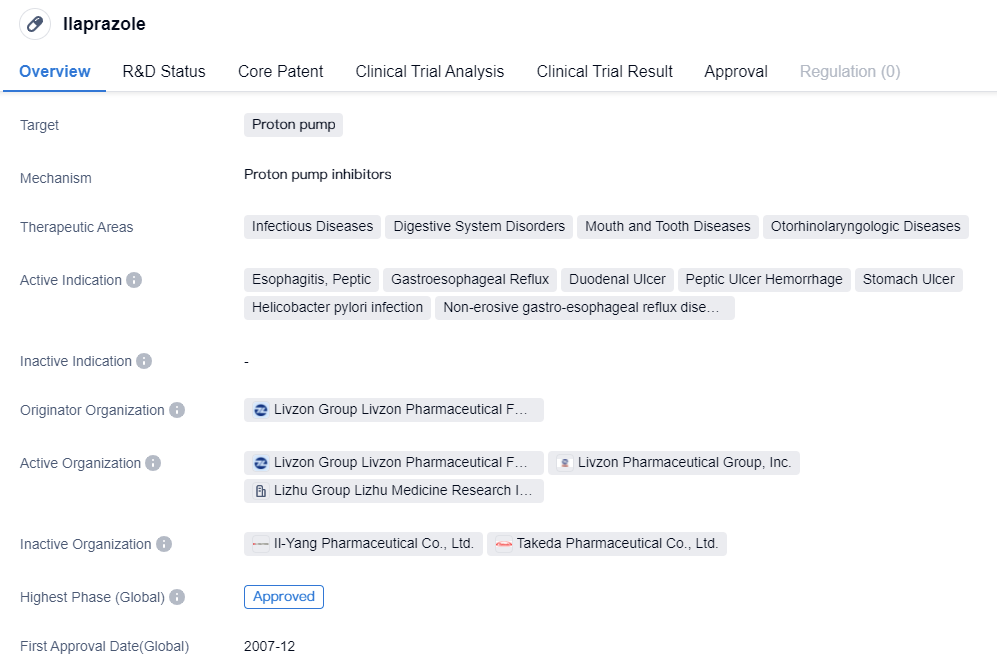
The CYP2C19-independent proton pump inhibitors include Rabeprazole Sodium and Ilaprazole, which are part of the second-generation proton pump inhibitors. Compared to the first-generation proton pump inhibitors, they have faster onset, better acid suppression, longer acid suppression time (24-hour acid suppression), no obvious breakthrough at night, less individual variation, high safety, and fewer drug interactions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Reversible Proton Pump Inhibitors belong to the Potassium-Competitive Acid Blocker (P-CAB) class, which competitively antagonizes the K+ binding sites on the proton pumps of gastric wall cells, inhibiting the exchange of cytoplasmic K+ and intracellular H+, thus achieving the purpose of acid suppression. They have a fast onset of action, strong acid suppression effect, higher selectivity and adverse reactions.