Corteria Pharmaceuticals Initiates Phase 1 Trial for Heart Failure Drug COR-1167
Corteria Pharmaceuticals, which is actively engaged in the advancement of innovative treatments for cardiovascular malfunctions and weight management issues, publicly declared the commencement of their Phase 1 clinical trial. This examination is designed to assess their pioneering compound, COR-1167, an activator of the corticotropin-releasing hormone receptor 2 (CRF2). The investigational drug is aimed at addressing patients with progressive deterioration in heart function.
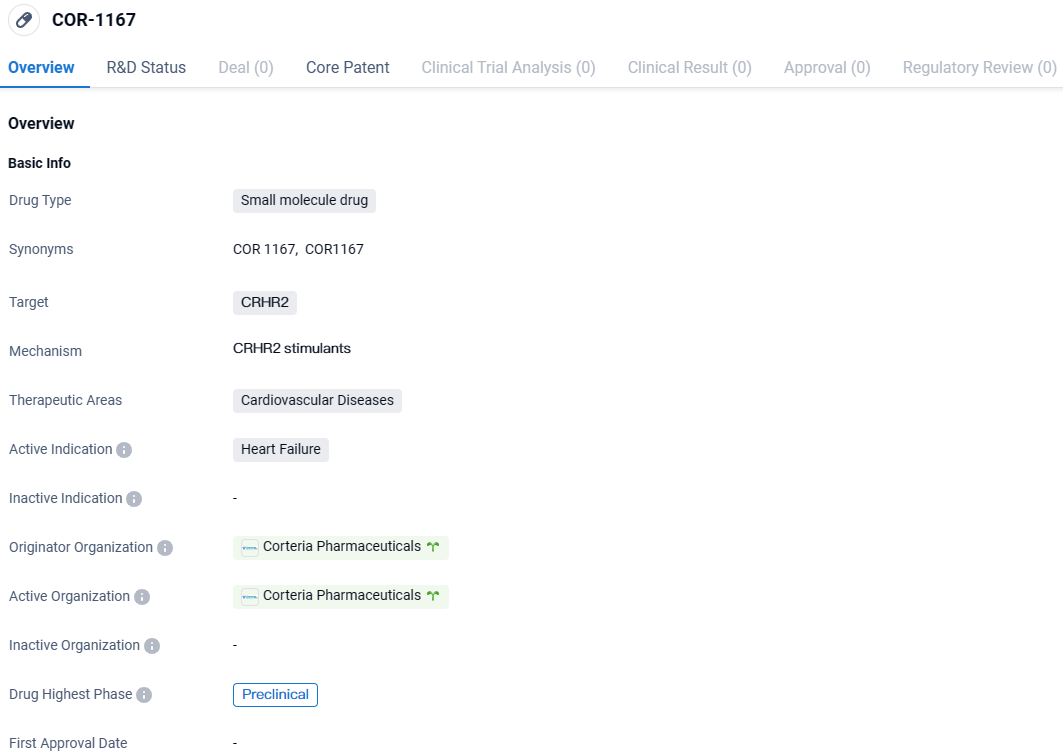
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
COR-1167 is administered once daily via a subcutaneous injection and functions as an activator of the CRF2 receptor. It has shown notable benefits, improving cardiac, vascular, and renal functions in a variety of heart failure animal studies. The distinctive way in which COR-1167 works presents a new therapeutic angle to address WHF; its primary therapeutic aim is to relieve heart stress by preserving and enhancing circulatory and kidney stability.
The initial phase of the clinical trials involves a study where the participants are randomly assigned to either receive COR-1167 or a placebo, with the key goal being to evaluate the compound’s safety and the body's ability to tolerate it in both individuals who are healthy and those living with chronic heart failure.
“The progression of our preliminary CRF2 receptor activator to the clinical trial phase marks a pivotal development in our pursuit of alternative treatments for patients suffering from WHF,” Philip Janiak, the creator and chief executive of Corteria Pharmaceuticals, articulated. “The data obtained from preclinical research on COR-1167 looks highly encouraging, and we're eager to assess its potential to treat patients.”
Prof. Adriaan Voors, a cardiology expert at the University Medical Centre Groningen and the chief researcher for this trial, highlighted the significance of this Phase 1 trial which focuses on COR-1167: “Implementing this clinical trial for a CRF2 receptor activator in both healthy individuals and those suffering from heart failure marks a crucial development towards lessening the impact of heart failure. This is especially vital for patients who still struggle with deteriorating conditions even after following existing recommended treatment protocols.”
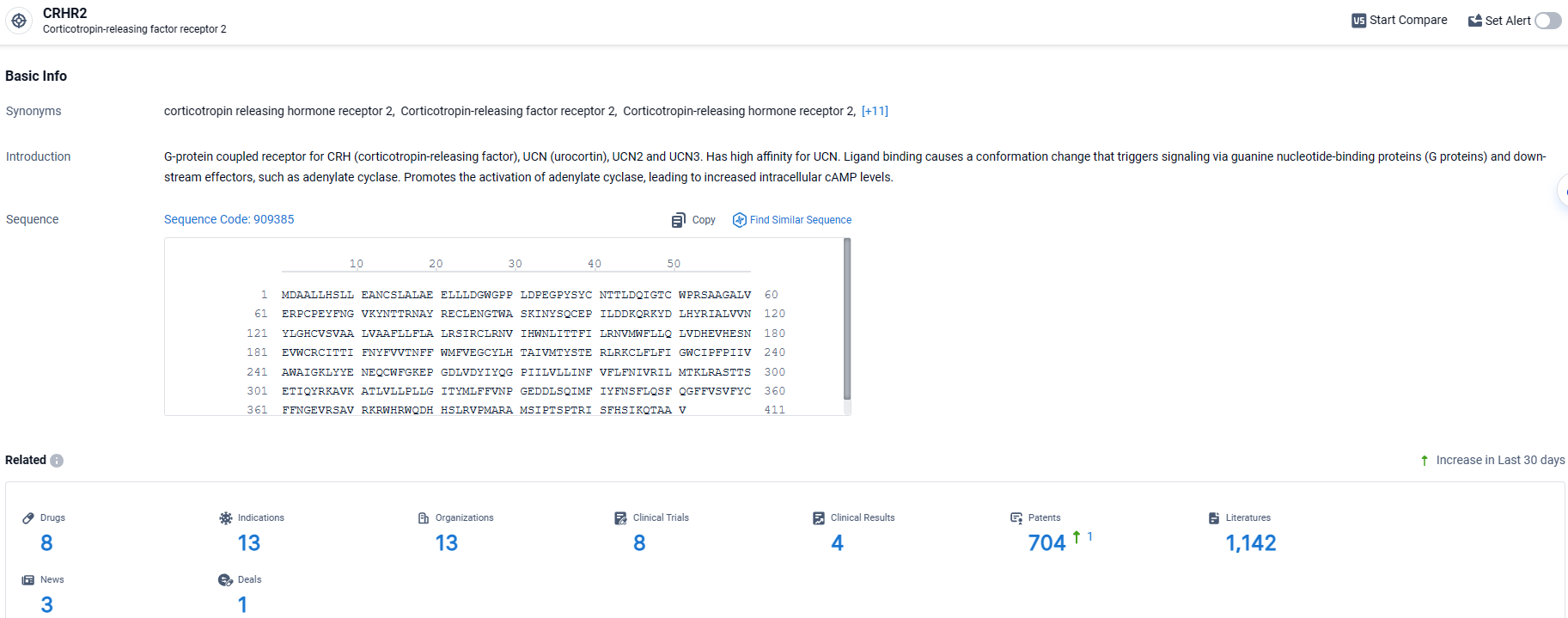
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 27, 2024, there are 8 investigational drugs for the CRHR2 target, including 13 indications, 13 R&D institutions involved, with related clinical trials reaching 8, and as many as 1142 patents.
COR-1167 targets CRHR2, a receptor involved in cardiovascular diseases. While still in the preclinical phase, the drug shows promise in potentially improving cardiac function and addressing the unmet medical need in heart failure. Further research and development will be necessary to determine its safety and efficacy in human clinical trials.