Intravacc reveals encouraging initial human trial results for its novel OMV nasal spray vaccine against COVID-19
Intravacc, a globally recognized pioneer in the field of translational research as well as the creation of vaccines for both prevention and treatment, has shared encouraging results from initial human trials of Avacc 10. This vaccine is designed as an intranasal booster, utilizing outer membrane vesicles, targeting the novel coronavirus, SARS-CoV-2. The foundational goals of this first-in-human trial included establishing the safe use, acceptable tolerance levels, and the ability to provoke an immune response when Avacc 10 is delivered through the nasal passage.
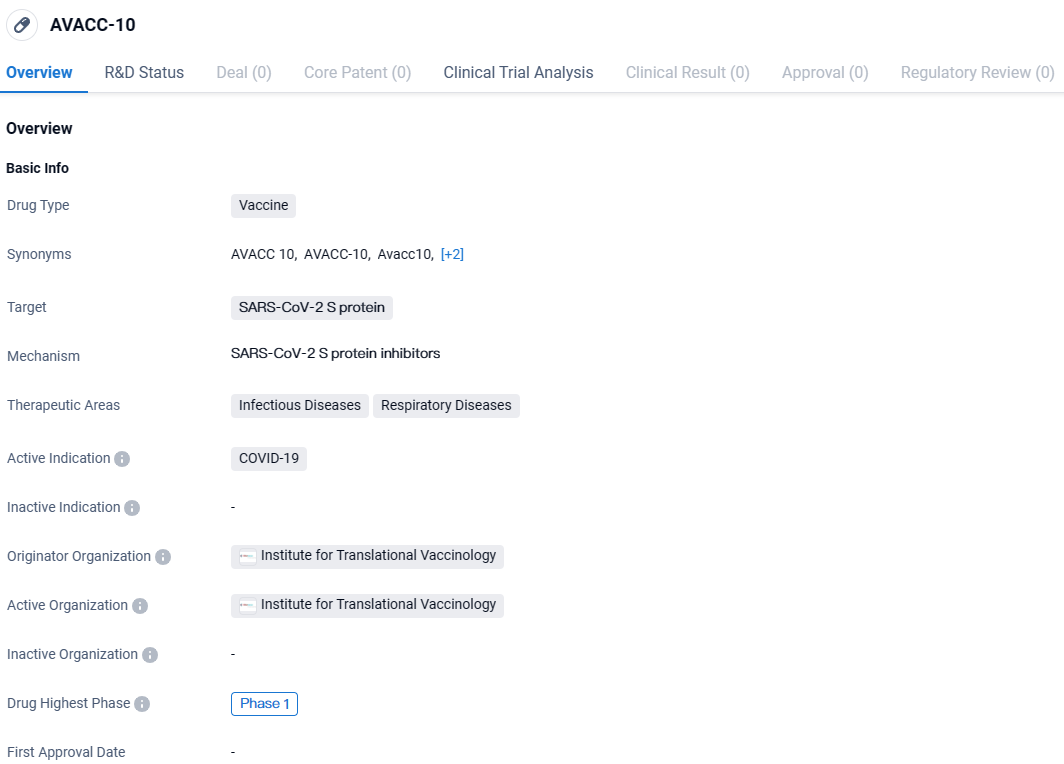
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
A clinical investigation's initial phase, executed in the Australian setting, concentrated on determining the acceptability, security, and immune response eliciting capacity of the Avacc 10 inoculation. The experiment employed a structured process characterized by random assignment, double-blind secrecy, and a control involving placebos as well as Outer Membrane Vesicles. It involved the participation of 36 robust individuals - an assembly of men and women between 18 to 55 years. Each participant was administered a duo of nasal sprays containing the Avacc 10 formulation, separated by a three-week interval. Subdividing the participants, one cohort received a lesser quantity while another was given a greater quantity of the Avacc 10 dosage.
During the ensuing half-year after the administration of the vaccine, both cohorts were under observation. The investigative effort further included gauging Avacc 10's effectiveness in prompting an immunological reaction by examining specific immune elements like IgA and IgG, as well as antibodies with virus-neutralizing capabilities, particularly in those volunteers who already had considerable amounts of IgG antibodies directed against the SARS-CoV-2 virus.
The administration of the Avacc 10 inoculation proceeded without any noted incidents of negative reactions that might have necessitated the cessation of a participant's involvement in the study. Notably, the immunological engagement findings for the cohort which had received the amplified dosage of Avacc 10 showcased an initiation of an immune response within the mucous membranes.
Intravacc's Lead Scientist for Research & Development, Dr. Dinja Oosterhoff, commented: “The positive outcomes reflecting the immune activation from our Phase 1 assessment of Avacc 10® decidedly affirm the potential of an OMV-bolstered vaccine in enhancing the body's immune defenses.”
Moreover, Intravacc’s principal executive, Dr. Jan Groen, elaborated: “The initialization of this first-in-human (FIH) intranasal vaccine trial is a crucial advancement for Intravacc's primary OMV-dependent vaccine technology. As part of expanding our technology's purview, Intravacc is embarked on formulating an intranasal solution targeting Gonorrhea set for its first human trials in the last quarter of 2025. Concurrently, Intravacc has successfully entered into licensing agreements for its OMV-dependent vaccines with major pharmaceutical entities.”
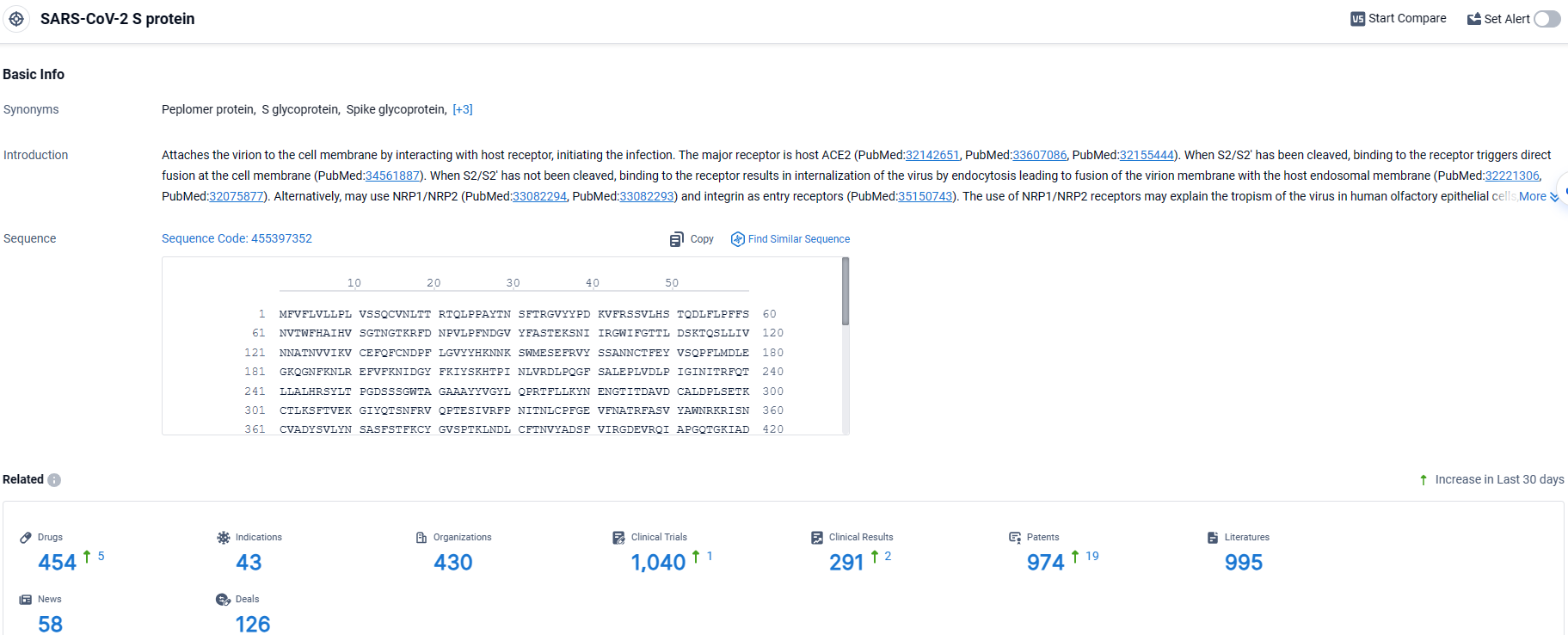
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 27 2024, there are 454 investigational drugs for the SARS-CoV-2 S protein target, including 43 indications, 430 R&D institutions involved, with related clinical trials reaching 1040, and as many as 974 patents.
AVACC-10 is a vaccine developed by the Institute for Translational Vaccinology that specifically targets the SARS-CoV-2 S protein. It is intended to be used for the prevention and treatment of COVID-19, particularly in the context of infectious and respiratory diseases. Currently, AVACC-10 is in Phase 1 of its development, indicating that it has undergone initial testing in humans to assess its safety and dosage.