CV6 Therapeutics Begins Dosing Patients in Phase 1a Trial for Novel dUTPase Inhibitor CV6-168
CV6 Therapeutics (NI) Ltd. ("CV6"), a clinical-stage pharmaceutical firm focused on enhancing patient outcomes in cancer and inflammatory diseases through the development of innovative, first-in-class small molecules that target Uracil-DNA metabolism, has reported the successful administration of the second patient in its Phase 1a clinical trial assessing CV6-168 in combination with infusional 5-fluorouracil (5-FU) for cancer treatment.
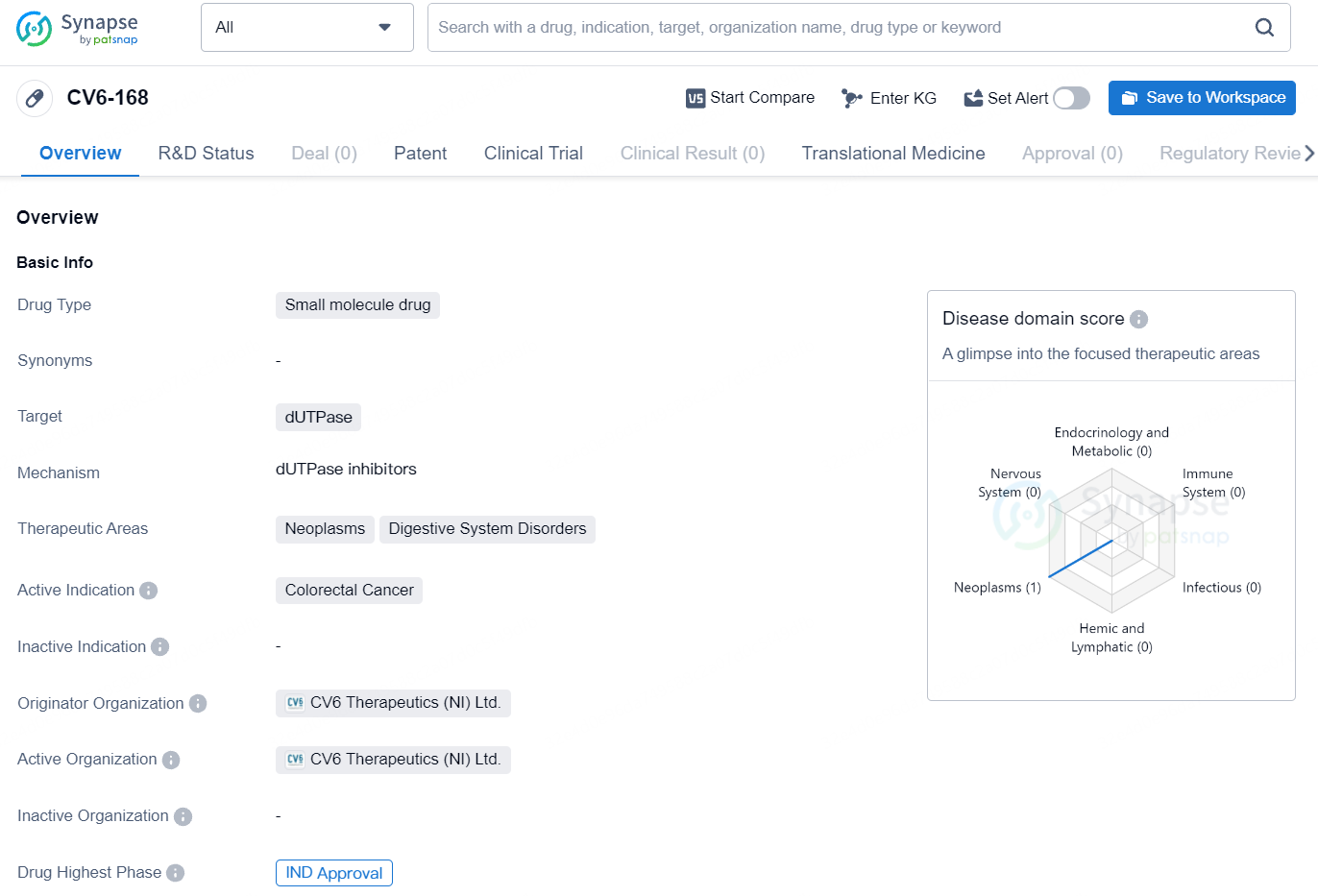
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 1 segment of this modular, first-in-human Phase 1a clinical study (ISRCTN12434145) is a multicenter, open-label, dose-escalation trial of CV6-168 targeting patients with advanced metastatic solid tumors that have not responded to standard therapies. The main goals of the study include assessing the safety profile of CV6-168, identifying potentially optimal biologically relevant doses, determining the maximum tolerated dose, and evaluating its anti-tumor efficacy.
"CV6-168 initiates DNA Uracilation, a breakthrough mechanism of action, and represents our inaugural drug candidate to advance to clinical trials. CV6-168 holds promise for treating a range of frequently diagnosed cancers when combined with key therapies that are administered to millions of patients annually worldwide," stated Dr. Robert D. Ladner, the founder and CEO of CV6.
"CV6-168 enhances the clinical translation of Uracil-DNA biology by causing the erroneous incorporation of uracil into the DNA of cancer cells, which leads to uracil-repair-mediated DNA damage, immune modulation, and cell death. The administration of the second patient in our initial clinical trial signifies a crucial milestone as CV6 strives to provide innovative and potent first-in-class small molecules with a novel mechanism of action for patients dealing with prevalent, difficult-to-treat cancers."
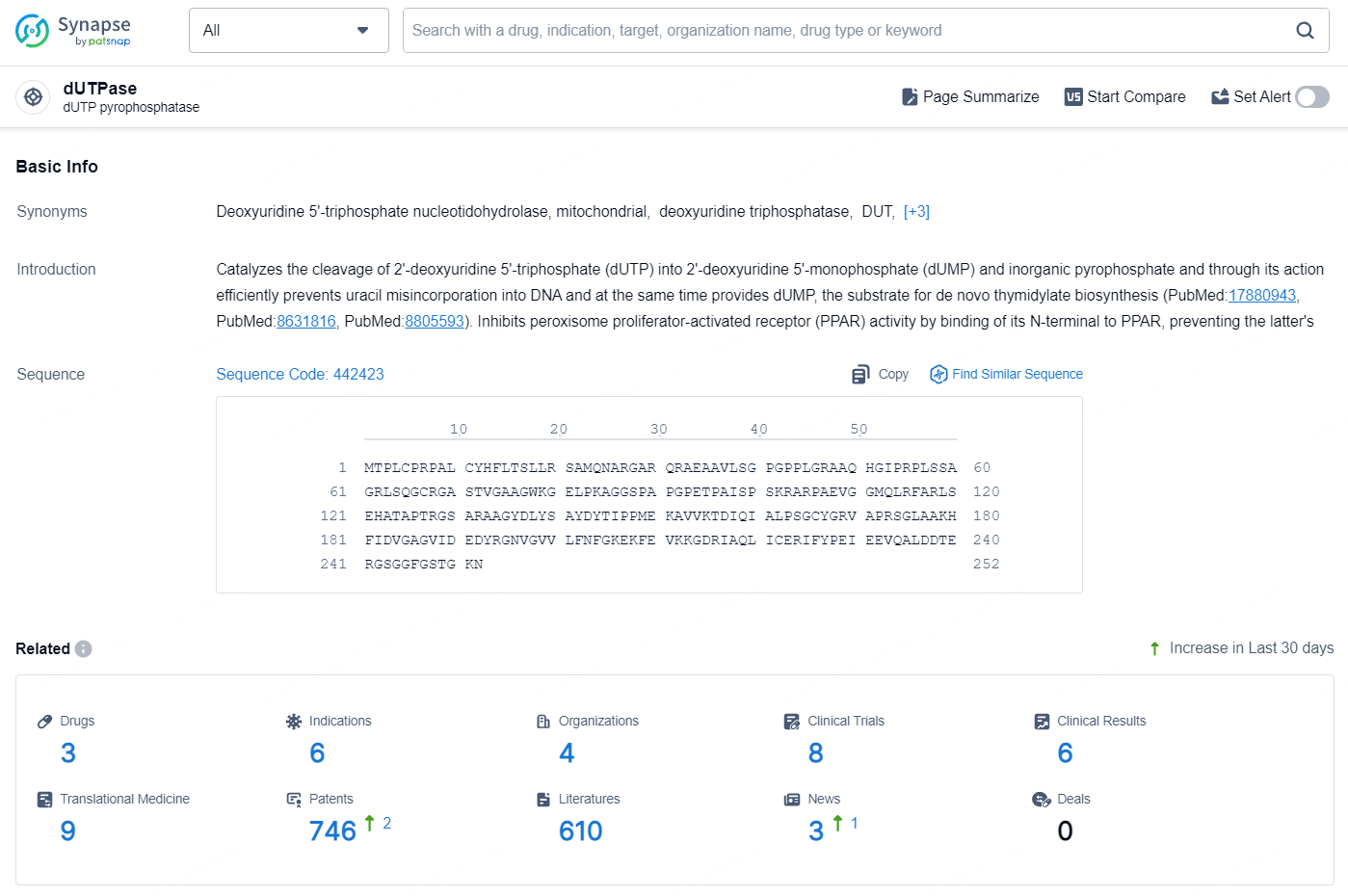
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 19, 2024, there are 3 investigational drugs for the dUTPase target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 8, and as many as 746 patents.
CV6-168 is a novel, targeted, small molecule, dUTPase-specific inhibitor in development for the treatment of multiple, high-incidence cancers. In preclinical studies, it has demonstrated robust anti-tumor activity in cell line and animal models with no added toxicity. CV6-168 targets the nucleotide metabolism enzyme dUTPase, with high specificity, avoiding drug-drug interactions with its drug combination partners that include thymidylate synthase (TS) inhibitors like 5-fluorouracil (5-FU).