UroGen Submits UGN-102 NDA, Targets FDA Approval for Intermediate-Risk Bladder Cancer Treatment
UroGen Pharma Ltd. (Nasdaq: URGN), a prominent biotechnology firm focused on pioneering therapies for urothelial and specialty cancers, has successfully finalized the submission of its New Drug Application (NDA) for the experimental drug UGN-102, (mitomycin) for intravesical solution. This accomplishment represents a pivotal move towards potentially meeting the critical demand for groundbreaking treatments for low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC). UroGen foresees the possibility of obtaining FDA approval by early 2025, provided the NDA is accepted for review by the FDA and granted priority review status.
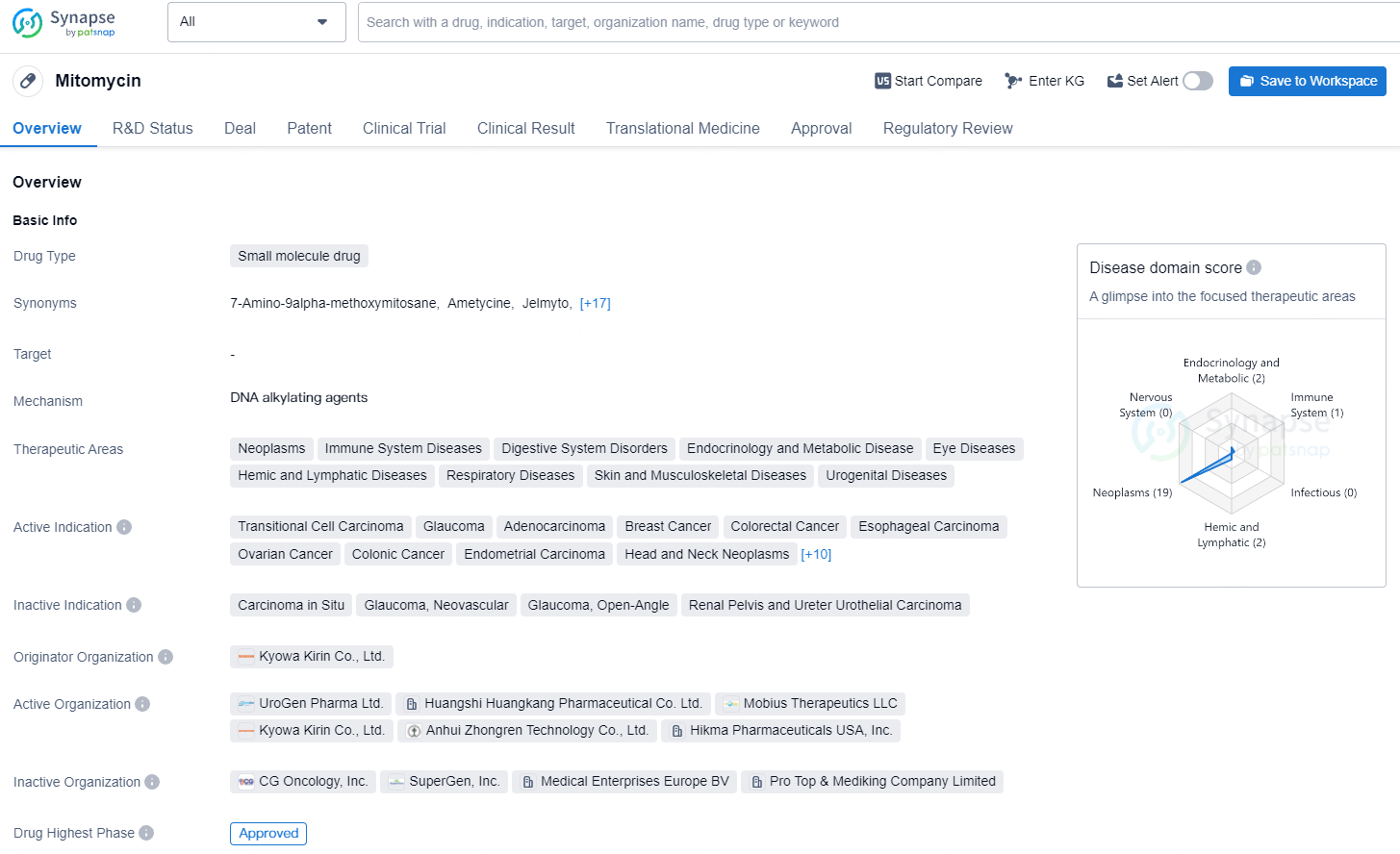
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 "The submission of the NDA for UGN-102 signifies an essential milestone for UroGen, highlighting our commitment to progressing this innovative treatment for patients with LG-IR-NMIBC," stated Liz Barrett, President and CEO of UroGen. "UGN-102, if approved, may present a viable alternative to repeated surgeries, potentially providing patients with substantial quality of life improvements and significant recurrence-free periods. Given the high recurrence rates of LG-IR-NMIBC, the urgency for novel therapies like UGN-102 is critical. It has the potential to become a crucial new option for managing this difficult condition."
"The submission of the NDA for UGN-102 signifies an essential milestone for UroGen, highlighting our commitment to progressing this innovative treatment for patients with LG-IR-NMIBC," stated Liz Barrett, President and CEO of UroGen. "UGN-102, if approved, may present a viable alternative to repeated surgeries, potentially providing patients with substantial quality of life improvements and significant recurrence-free periods. Given the high recurrence rates of LG-IR-NMIBC, the urgency for novel therapies like UGN-102 is critical. It has the potential to become a crucial new option for managing this difficult condition."
The NDA is bolstered by the clinical program for UGN-102, which includes long-term durability results from the Phase 3 ENVISION study. The ENVISION study reached its primary endpoint, showing a 79.6% complete response (CR) rate at three months post-initial instillation in patients treated with UGN-102. The 12-month duration of response (DOR) for patients achieving a CR at three months was estimated at 82.3% by Kaplan-Meier analysis (n=108). DOR estimates for 15 months (n=43) and 18 months (n=9) post 3-month CR both stood at 80.9%.
In the ENVISION trial, the most frequently reported treatment-emergent adverse events (TEAEs) were dysuria, hematuria, urinary tract infection, pollakiuria, fatigue, and urinary retention. These TEAEs were generally mild to moderate in severity and were resolving or had been resolved. The safety profile observed in the ENVISION trial was consistent with that reported in other UGN-102 studies.
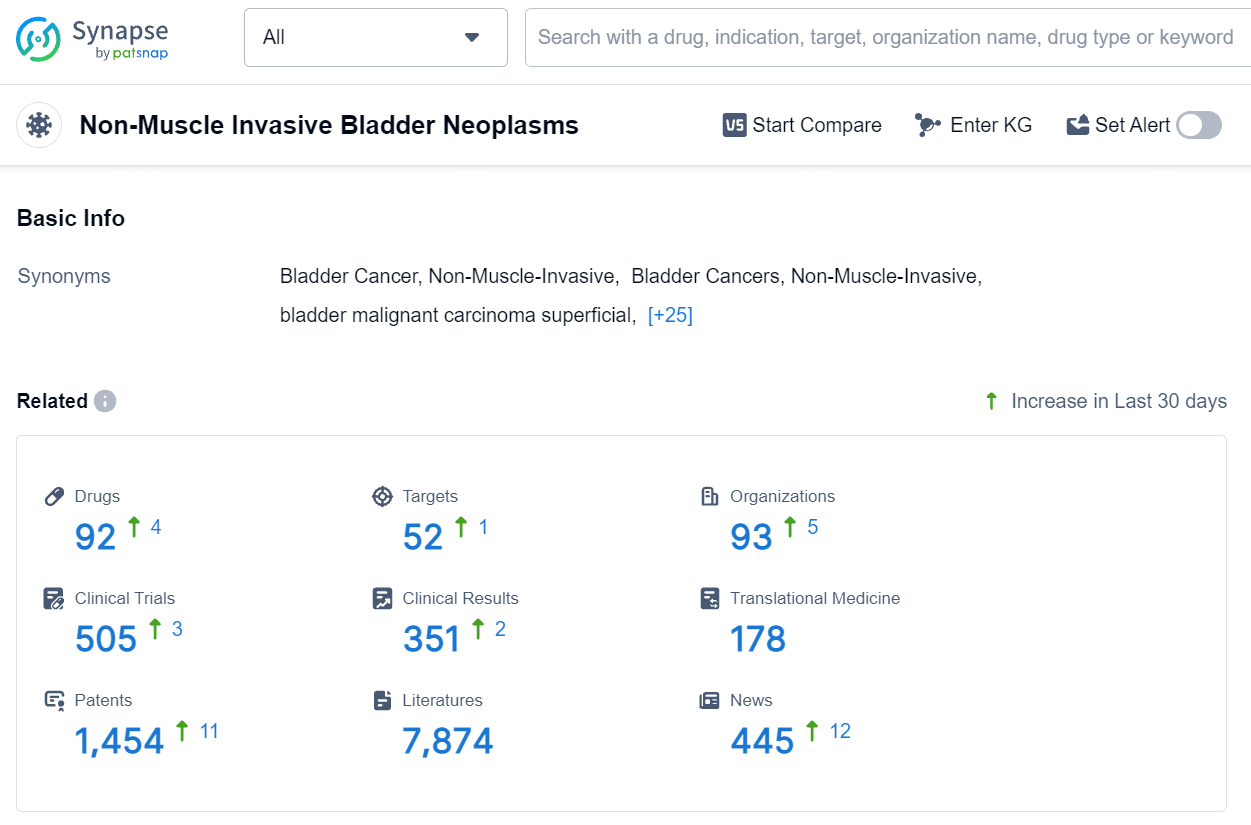
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 15, 2024, there are 92 investigational drugs for the non-muscle invasive bladder cancer, including 52 targets, 93 R&D institutions involved, with related clinical trials reaching 505, and as many as 1454 patents.UGN-102 (mitomycin) for intravesical solution is a novel drug formulation of mitomycin, presently in Phase 3 clinical trials for LG-IR-NMIBC treatment. Leveraging UroGen's proprietary RTGel® technology, which is a hydrogel-based sustained release formulation, UGN-102 is intended to prolong the exposure of bladder tissue to mitomycin. This facilitates tumor treatment without the need for surgery. Administration of UGN-102 is carried out by a trained healthcare professional using a standard urinary catheter in an outpatient context.