Cystic Fibrosis Treatment Drug - CFTR Modulators
Cystic Fibrosis (CF) is a multisystem autosomal recessive disease, caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. The CFTR protein is an ion channel mainly used to regulate the transepithelial movement of anions in mucus secretion tissues, thereby coordinating the balance of salt and fluid levels in mucus secretions. Pathogenic mutations in this gene leading to CF can affect multiple organs including the intestines, pancreas, and liver, with the dysfunction of CFTR in the airways particularly detrimental to CF patients. Dehydration of airway surface liquid and thickening of mucus secretions hinder effective airway mucociliary clearance, providing fertile ground for harmful bacteria and leading to repeated infections throughout a patient's life.
CFTR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 10 Sep 2023, there are a total of 87 CFTR drugs worldwide, from 69 organizations, covering 27 indications, and conducting 316 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.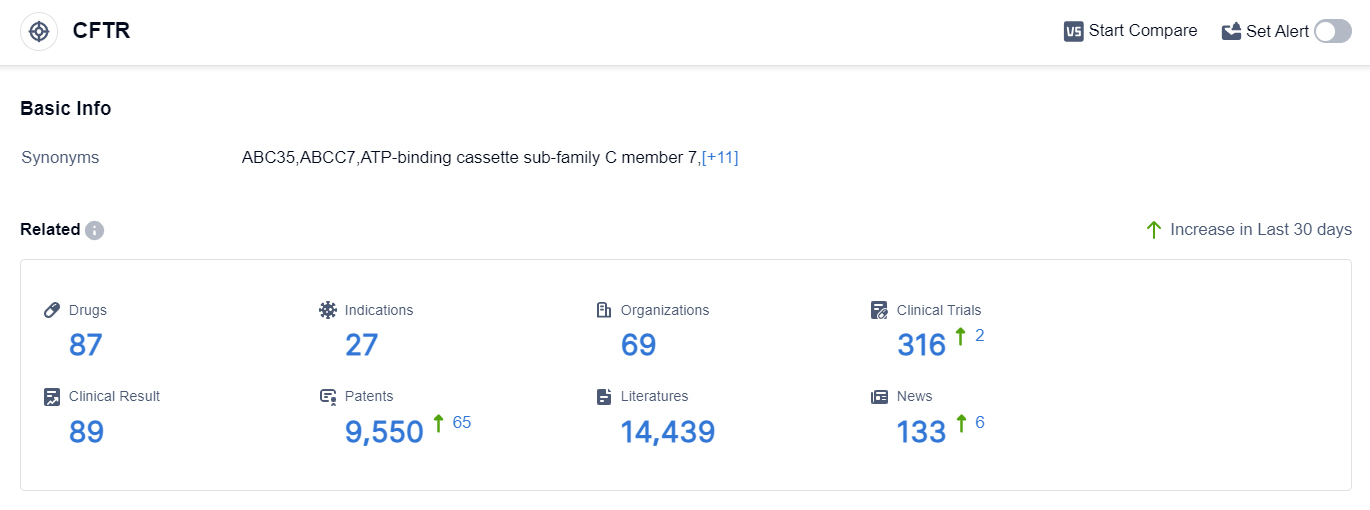
The analysis of the target CFTR reveals a competitive landscape with Vertex Pharmaceuticals, Inc. leading in terms of company growth and R&D progress. Cystic Fibrosis is the primary indication for drugs under this target, with several drugs approved and ongoing research in Preclinical and Inactive stages. Small molecule drugs are progressing rapidly, indicating intense competition in this area.
The United States is at the forefront of CFTR-related drug development, followed by the European Union and other countries. Overall, the target CFTR shows promising potential for the development of treatments for CFTR-related conditions, with ongoing research and development efforts by various companies and countries.
CFTR Modulators Entering Phase III Clinical Trials: Vanzacaftor
Vanzacaftor is a small molecule drug developed by Vertex Pharmaceuticals, Inc. It falls under the therapeutic areas of congenital disorders, digestive system disorders, and respiratory diseases. The drug specifically targets CFTR, which stands for Cystic Fibrosis Transmembrane Conductance Regulator.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Vanzacaftor is being developed as a potential treatment for CF. It is currently in Phase 3 of clinical development, which indicates that it has already undergone extensive testing in preclinical and early clinical trials. Phase 3 trials involve a larger number of participants and aim to evaluate the drug's safety and efficacy in a real-world setting.
As a small molecule drug, Vanzacaftor is likely to be orally administered. Small molecules are typically designed to interact with specific targets within cells, such as proteins or enzymes, to modulate their activity. In the case of Vanzacaftor, it is designed to target CFTR and potentially restore its function or improve its activity.
Vertex Pharmaceuticals, Inc. is the originator organization behind Vanzacaftor. They are a renowned pharmaceutical company specializing in the development of innovative therapies for serious diseases, including CF. With their expertise and resources, Vertex Pharmaceuticals is dedicated to advancing the treatment options available for patients with CF.
In summary, Vanzacaftor is a small molecule drug developed by Vertex Pharmaceuticals, Inc. It targets CFTR and is being developed as a potential treatment for Cystic Fibrosis. The drug falls under the therapeutic areas of congenital disorders, digestive system disorders, and respiratory diseases. Currently in Phase 3 of clinical development, Vanzacaftor holds promise for improving the lives of individuals affected by CF.
CFTR Modulators Entering Phase II Clinical Trials:ABBV-119
ABBV-119 is a small molecule drug that is being developed for the treatment of CF. The drug targets the CFTR protein, which is responsible for regulating the movement of salt and water in and out of cells.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, ABBV-119 is in Phase 2 of clinical development, which means that it has already undergone initial testing in humans to evaluate its safety and efficacy. Phase 2 trials typically involve a larger number of participants and aim to further assess the drug's effectiveness and side effects. This phase is crucial in determining whether the drug has the potential to be a viable treatment option for CF patients.
The therapeutic areas of ABBV-119 include Congenital Disorders, Digestive System Disorders, and Respiratory Diseases. This suggests that the drug may have the potential to address not only CF but also other conditions related to these therapeutic areas. However, it is important to note that the drug's primary indication is for the treatment of CF.
As a small molecule drug, ABBV-119 is likely to have a different mechanism of action compared to biologic drugs. Small molecules are typically orally available and can be easily synthesized, making them more convenient for patients and potentially more cost-effective. However, they may also have limitations in terms of specificity and potency compared to biologics.
In summary, ABBV-119 is a small molecule drug that is currently in Phase 2 of clinical development for the treatment of Cystic Fibrosis. It targets the CFTR protein and has potential therapeutic applications in Congenital Disorders, Digestive System Disorders, and Respiratory Diseases. Further research and clinical trials are needed to determine the drug's efficacy and safety profile.