Decoding Casirivimab: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Casirivimab's R&D Progress
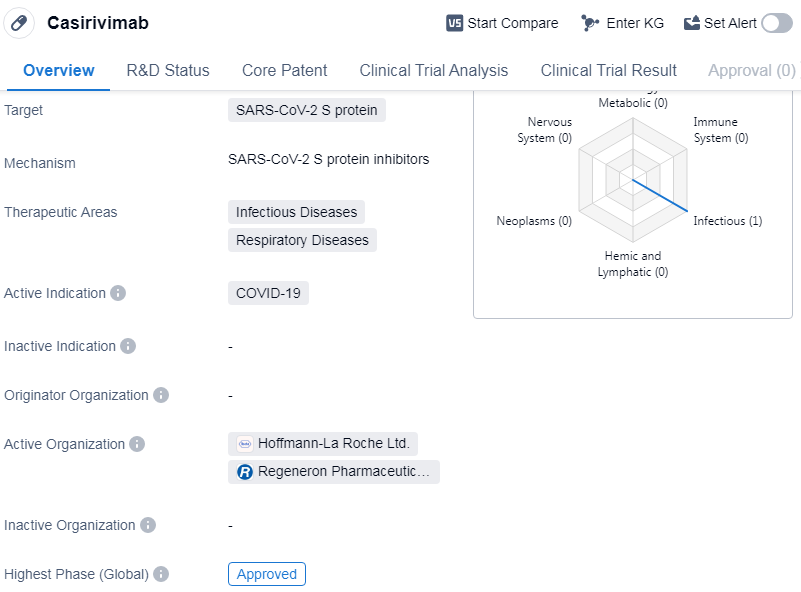
Casirivimab is a monoclonal antibody drug that targets the SARS-CoV-2 S protein. It falls under the therapeutic areas of Infectious Diseases and Respiratory Diseases, with its active indication being COVID-19. The drug received its highest phase of approval, indicating that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
The first approval of Casirivimab took place in the United States in November 2020. As a monoclonal antibody, Casirivimab is designed to mimic the natural antibodies produced by the immune system to fight off infections. By targeting the S protein of the SARS-CoV-2 virus, it aims to neutralize the virus and potentially reduce the severity of COVID-19 symptoms.
Casirivimab's approval in the United States suggests that it has met the necessary safety and efficacy standards set by regulatory authorities.
The approval of Casirivimab also highlights the importance of targeted therapies in the field of biomedicine. By specifically targeting the S protein of the SARS-CoV-2 virus, Casirivimab aims to disrupt the virus's ability to enter and infect human cells.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Casirivimab: SARS-CoV-2 S protein inhibitors
From a biomedical perspective, SARS-CoV-2 S protein inhibitors refer to substances or drugs that are designed to inhibit or block the activity of the S protein of the SARS-CoV-2 virus. The S protein, also known as the spike protein, is a key component of the virus responsible for facilitating its entry into human cells. By inhibiting the S protein, these inhibitors aim to prevent viral attachment and entry into host cells, thereby potentially reducing viral replication and the severity of COVID-19. These inhibitors can be developed through various approaches, such as small molecules, peptides, or antibodies, that specifically target the S protein and interfere with its function.
Drug Target R&D Trends for Casirivimab
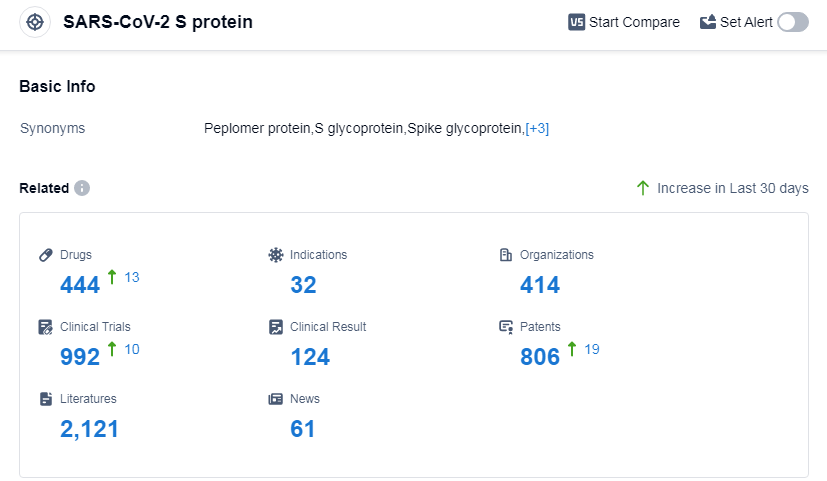
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 444 SARS-CoV-2 S protein drugs worldwide, from 414 organizations, covering 32 indications, and conducting 992 clinical trials.
The analysis of the target SARS-CoV-2 S protein reveals a competitive landscape with multiple companies actively involved in R&D. Moderna, Inc., Pfizer Inc., Regeneron Pharmaceuticals, Inc., and Roche Holding AG are among the companies with the highest stage of development. The most common indication for approved drugs is COVID-19. Prophylactic vaccines, RNA vaccines, and monoclonal antibodies are progressing rapidly. China has shown significant progress in the development of drugs targeting the SARS-CoV-2 S protein. Overall, the future development of this target is promising, with ongoing research and development efforts focused on addressing the COVID-19 pandemic and related indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Casirivimab is a monoclonal antibody drug that targets the S protein of the SARS-CoV-2 virus. It has received regulatory approval in the United States for the treatment of COVID-19. The drug's approval signifies an important milestone in the ongoing efforts to combat the pandemic and provides healthcare professionals with an additional therapeutic option. Further research and monitoring will be necessary to assess the long-term efficacy and safety of Casirivimab in treating COVID-19.