Decoding Pemafibrate: A Comprehensive Study of its R&D Trends
Pemafibrate's R&D Progress
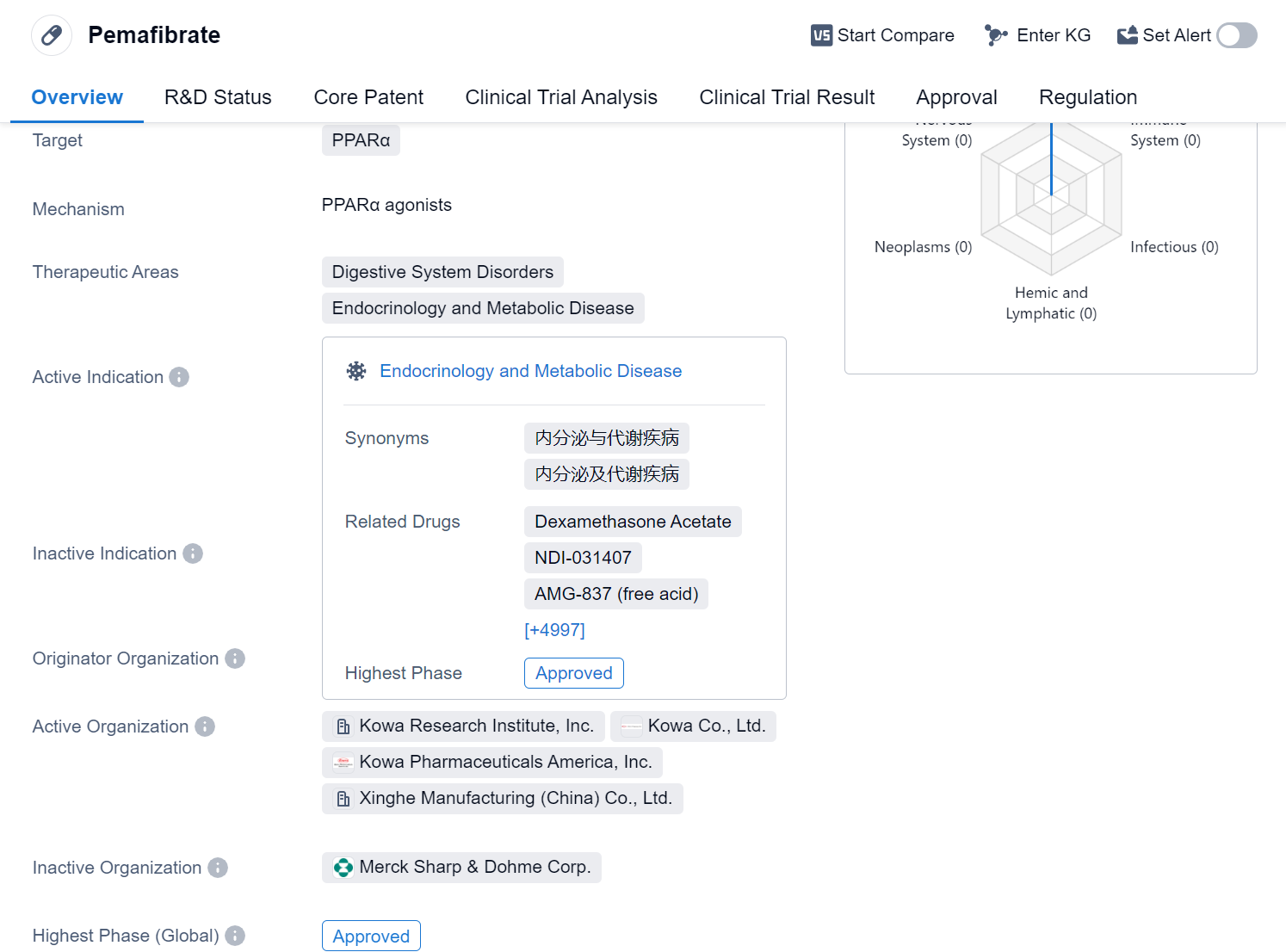
Pemafibrate is a small molecule drug that targets PPARα, a receptor involved in regulating lipid metabolism. It is primarily used in the treatment of various disorders related to the digestive system, endocrinology, and metabolic diseases. The drug has been approved for use in several indications, including hyperlipidemias, hypercholesterolemia, dyslipidemias, hypertriglyceridemia, nonalcoholic steatohepatitis, liver cirrhosis, biliary, and primary biliary cholangitis.
Pemafibrate was developed by Kowa Co., Ltd., a pharmaceutical company based in Japan. It received its first approval in July 2017 in Japan, making it available for use in the country. The drug has also undergone clinical trials in China, reaching phase 3, which indicates that it is in an advanced stage of development in the Chinese market.
One notable aspect of Pemafibrate is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the drug's manufacturer, such as extended market exclusivity and financial support for research and development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pemafibrate: PPARα agonists
PPARα agonists are a type of drug that activate the peroxisome proliferator-activated receptor alpha (PPARα) in the body. PPARα is a nuclear receptor that plays a crucial role in regulating lipid metabolism and inflammation. When activated by PPARα agonists, this receptor promotes the expression of genes involved in fatty acid oxidation, leading to increased breakdown of fatty acids and improved lipid metabolism.
From a biomedical perspective, PPARα agonists are used in the treatment of various metabolic disorders, particularly dyslipidemia (abnormal lipid levels) and hypertriglyceridemia (high levels of triglycerides in the blood). By activating PPARα, these drugs help to lower triglyceride levels and increase levels of high-density lipoprotein (HDL) cholesterol, which is considered "good" cholesterol. This can have beneficial effects on cardiovascular health and reduce the risk of conditions such as atherosclerosis and coronary artery disease.
Some examples of PPARα agonists include fenofibrate and gemfibrozil, which are commonly prescribed to patients with dyslipidemia. These drugs are typically taken orally and work by binding to PPARα receptors in various tissues, including the liver, skeletal muscle, and adipose tissue. By modulating gene expression and lipid metabolism, PPARα agonists contribute to the overall management of lipid disorders and help improve the lipid profile of individuals.
Drug Target R&D Trends for Pemafibrate
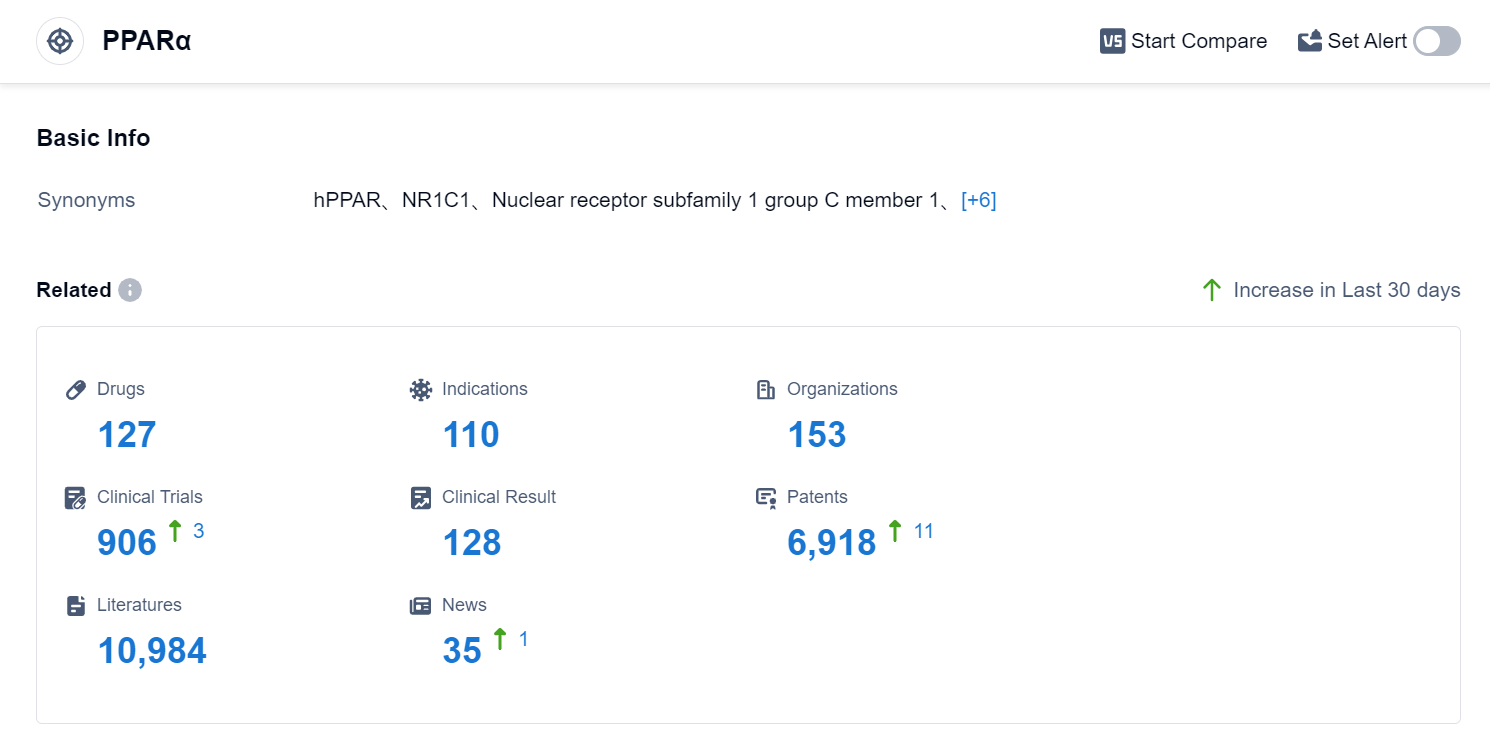
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 127 PPARα drugs worldwide, from 153 organizations, covering 110 indications, and conducting 906 clinical trials.
The analysis of the target PPARα reveals a competitive landscape with multiple companies growing rapidly under this target. The highest stage of development is the approved phase, indicating successful drug development in this area. The indications with the highest number of approved drugs include Hyperlipidemias, Hypertriglyceridemia, and Dyslipidemias. Small molecule drugs are progressing most rapidly, indicating a focus on this drug type. China, the European Union, the United States, and Japan are the countries/locations developing fastest under this target, with China showing significant progress. Overall, the target PPARα presents a competitive market with opportunities for further research and development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Pemafibrate represents a significant advancement in the field of biomedicine, particularly in the treatment of lipid-related disorders. As a small molecule drug targeting PPARα, it offers a potential therapeutic option for patients suffering from various digestive system disorders, endocrinology, and metabolic diseases. The drug's approval and orphan drug status highlight its potential to address unmet medical needs and improve patient outcomes in these areas.