The PCSK9 inhibitor is set to trigger a fierce competition in the hyperlipidemia market?
Hypercholesterolemia refers to a condition in which the cholesterol level in the blood is abnormally elevated. Cholesterol is a type of lipid substance that plays an important role in the human body, including the construction of cell membranes, hormone synthesis, and the production of bile acids, etc. When cholesterol levels in the blood persistently increase, it may increase the risk of cardiovascular diseases such as coronary heart disease, stroke, and atherosclerosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of hypercholesterolemia.
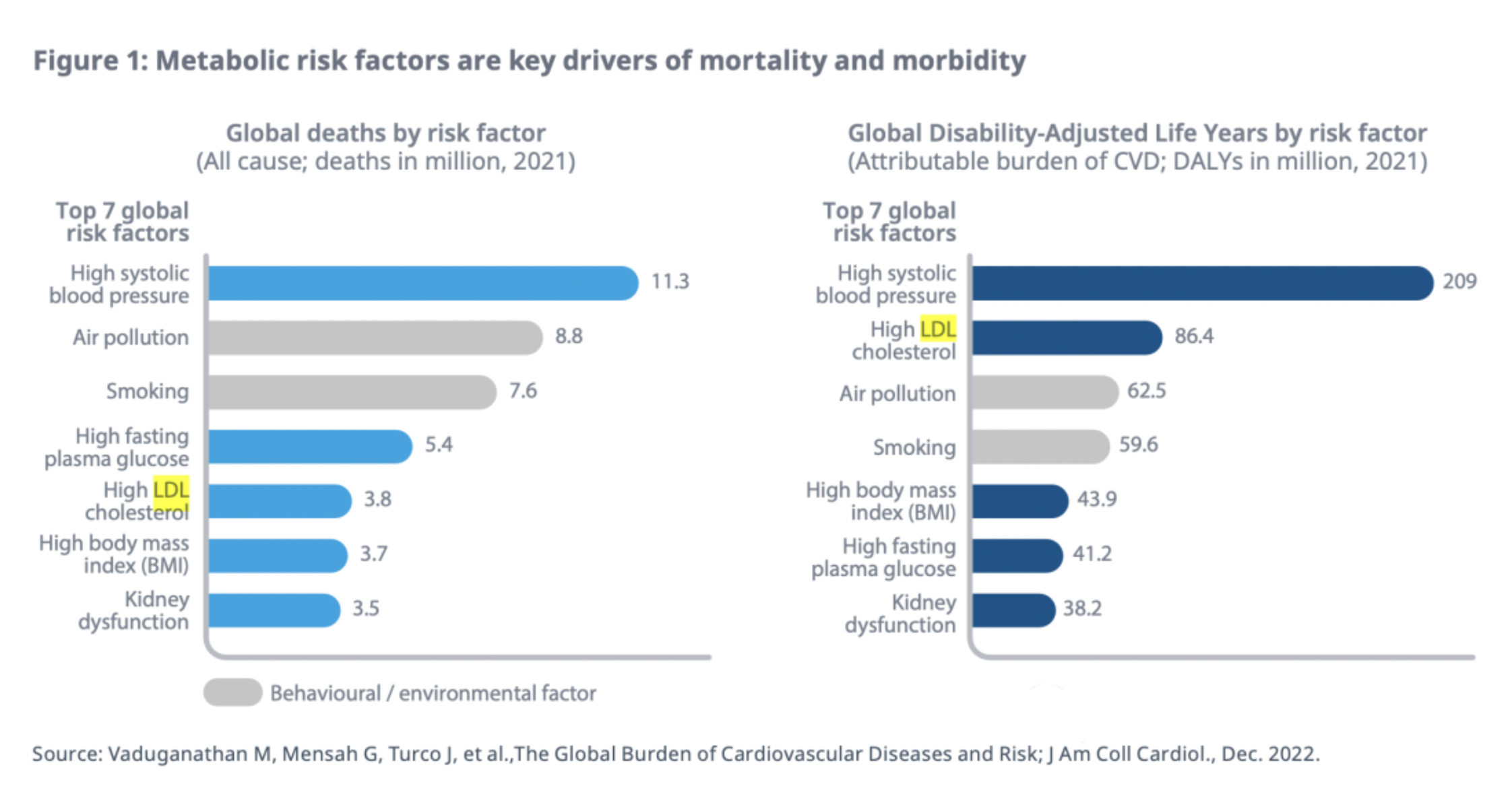
Since cholesterol is not soluble in water, it is transported in blood plasma through lipoprotein particles. Lipoproteins are classified by density into: Very Low-Density Lipoproteins (VLDL), Low-Density Lipoproteins (LDL), Intermediate-Density Lipoproteins (IDL), and High-Density Lipoproteins (HDL). All lipoproteins carry cholesterol, higher levels of High-Density Lipoprotein cholesterol have a protective effect, whereas an increase in Low-Density Lipoprotein cholesterol is associated with an increased risk of atherosclerosis and coronary heart disease.
High levels of Low-Density Lipoprotein Cholesterol (LDL-C) have become one of the major risk factors for mortality and morbidity worldwide.
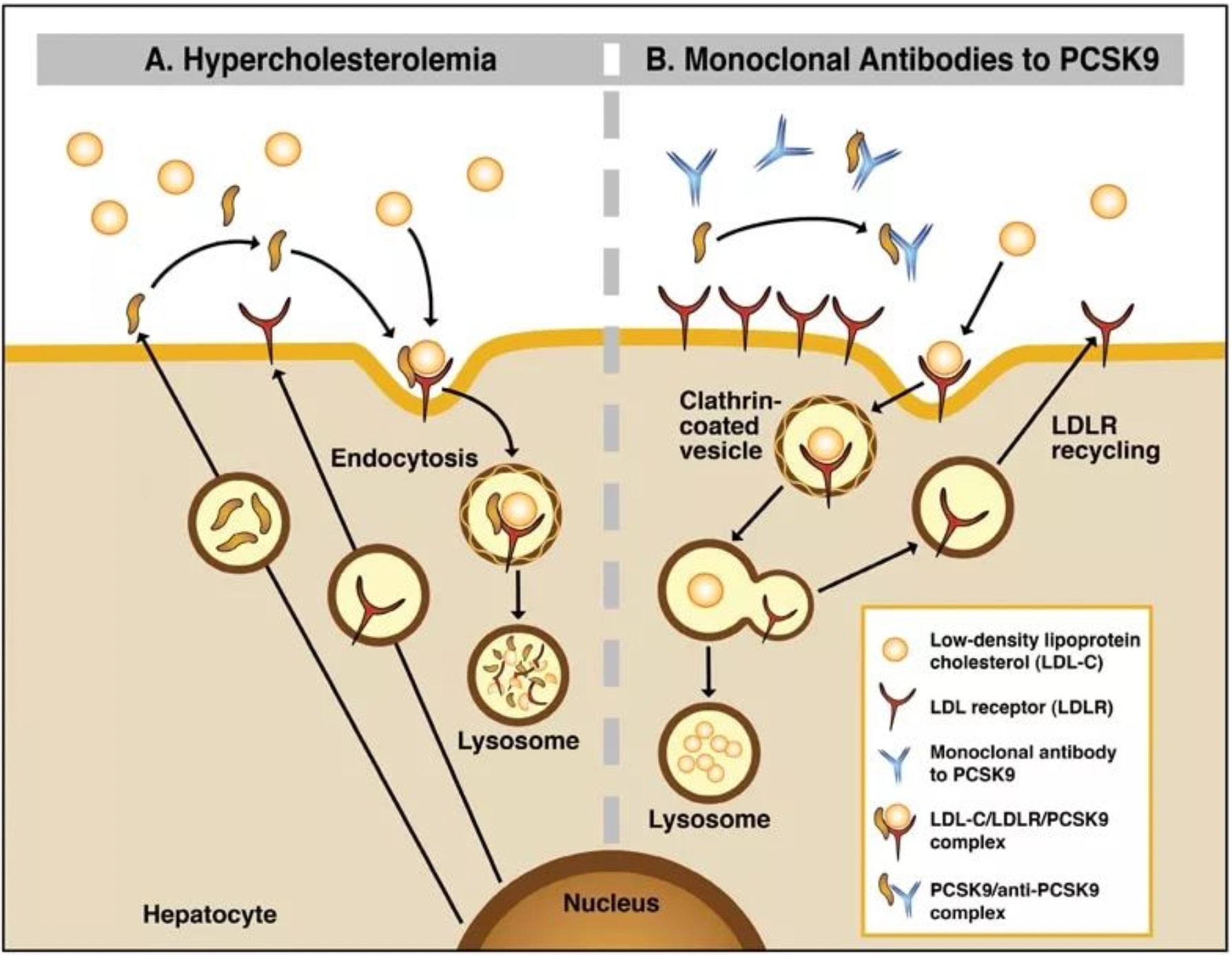
Under normal circumstances, low density lipoprotein receptors (LDLR) located on the surface of hepatic cells combine with APOB/LDL-C to form a complex, and through the interaction of LDLR and LDLRAP1, the complex is internalized into the cell. When the complex dissociates within the cell, LDLR are typically recycled to the cytoplasmic membrane, while free cholesterol is consumed within the cell. PCSK9 mediates the activity inhibition of LDLR through interactivity on the cell surface.
In hereditary hypercholesterolemia, single mutations in LDLR, APOB, PCSK9, and LDLRAP1 can lead to varying phenotypes ranging from mild to severe.
Mutations in LDLR can disrupt the binding of LDL-C to LDLR, resulting in the accumulation of LDL-C in the bloodstream. Mutations in the APOB gene can cause protein dysfunction, limiting the binding of LDL-C with LDLR.
Mutations in the PCSK9 gene can cause: (1) increased PCSK9 activity, (2) lack of LDLR recycling, (3) accumulation of LDL-C levels in the plasma. In such cases, anti-PCSK9 therapy, including emerging PCSK9 inhibitors (e.g., alirocumab, evolocumab), can be used to reduce LDL-C and prevent cardiovascular diseases.
Current Status of Drug Research Related to Hypercholesterolemia
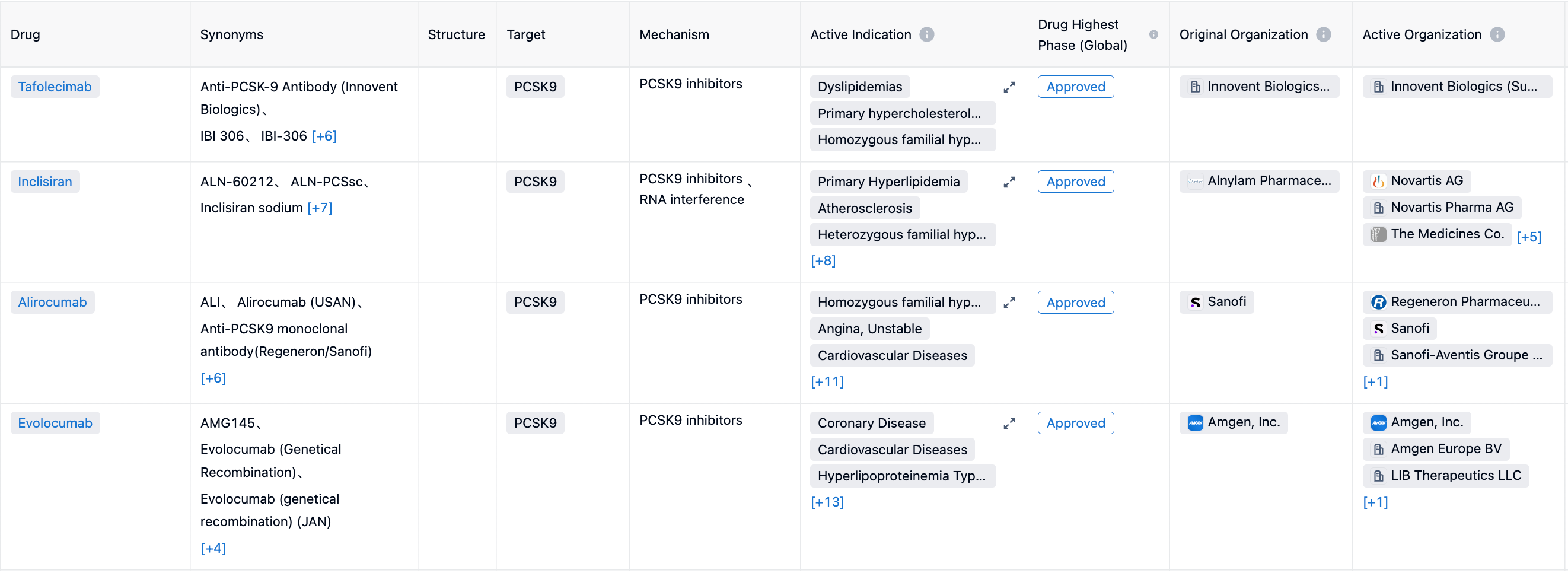
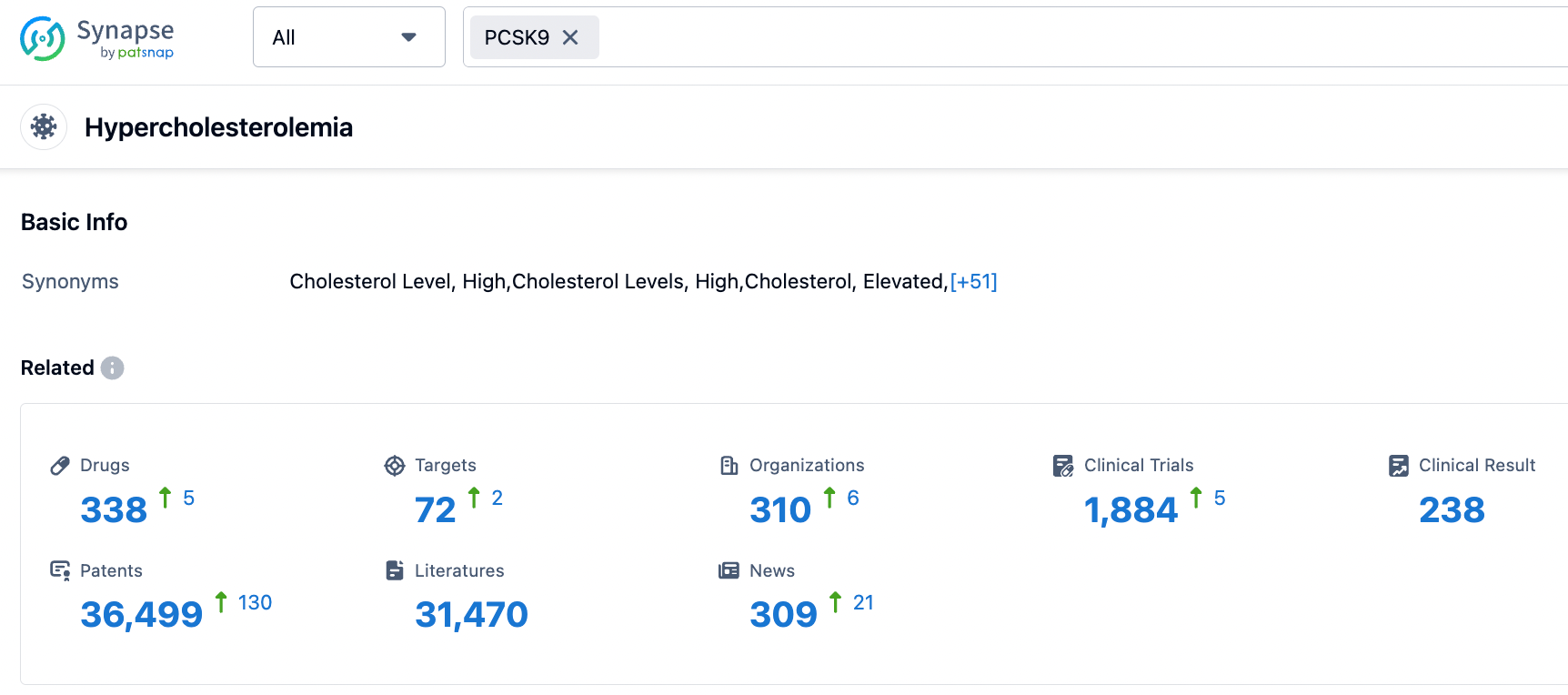
According to the Synapse database, hypercholesterolemia and its related metabolic diseases are popular research indications for global pharmaceutical companies. There are a total of 338 drugs for hypercholesterolemia, coming from 310 institutions, covering 72 targets, and conducting 1884 clinical trials.
Under the indication of hypercholesterolemia, HMG-CoA reductase is the most common clinical target, with 7 drugs already approved for marketing, and 20 drugs in the non-research stage. The second is PCSK9 target, with 4 approved drugs, 13 drugs in the preclinical stage, and 21 drugs in the non-research stage.
Using targeted PCSK9 drugs as examples, including the Tafolecimab from Innovent Biologics, Inclisiran from Novartis, Alirocumab from Sanofi and Regeneron, and Evolocumab from Amgen, have all been approved for marketing for diseases related to hypercholesterolemia or lipid disorders. Additionally, several drugs are in the stages of market application.
Novartis Company's small interfering RNA drug: Inclisiran
On August 22, Novartis's Inclisiran, a long-acting lipid-lowering small interfering RNA (siRNA) cholesterol-lowering drug, was approved by China's National Medical Products Administration for the treatment of adult primary hypercholesterolemia or mixed dyslipidemia.
Inclisiran is currently the first and only siRNA therapy in the world used to lower low-density lipoprotein cholesterol (LDL-C), where patients need only two injections per year to reduce LDL-C levels. Thanks to the long-lasting effects of the drug, patients do not need to take medication every day, earning Inclisiran the nickname of a "vaccine" in the cardiovascular field.
On August 28, Novartis's official website released the latest long-term data from ORION-8, which is the phase III open-label extension trial of the ORION-9, ORION-10, ORION-11, and ORION-3 trials. These data suggest that Leqvio (Inclisiran) can sustainably reduce low-density lipoprotein cholesterol (LDL-C) in patients with atherosclerotic cardiovascular disease (ASCVD) or heterozygous familial hypercholesterolaemia (HeFH) by administering twice a year, on top of statin therapy, and can last for more than six years.

Merck's Oral PCSK9 Inhibitor: MK-0616
On August 25, Merck announced on its official website the official launch of a Phase 3 clinical programme, CORALreef. This programme aims to investigate MK-0616, an oral PCSK9 inhibitor under investigation, for the treatment of Hypercholesterolemia in adults. Reportedly, this is the first Phase 3 clinical programme for an oral PCSK9 inhibitor.

Currently, the first batch of participants are taking part in two registration Phase 3 studies evaluating MK-0616's ability to reduce Low-Density Lipoprotein (LDL-C): CORALreef Lipids and CORALreef HeFH. Furthermore, Merck plans to launch a Phase 3 cardiovascular outcomes study -- CORALreef Outcomes, by the end of 2023.