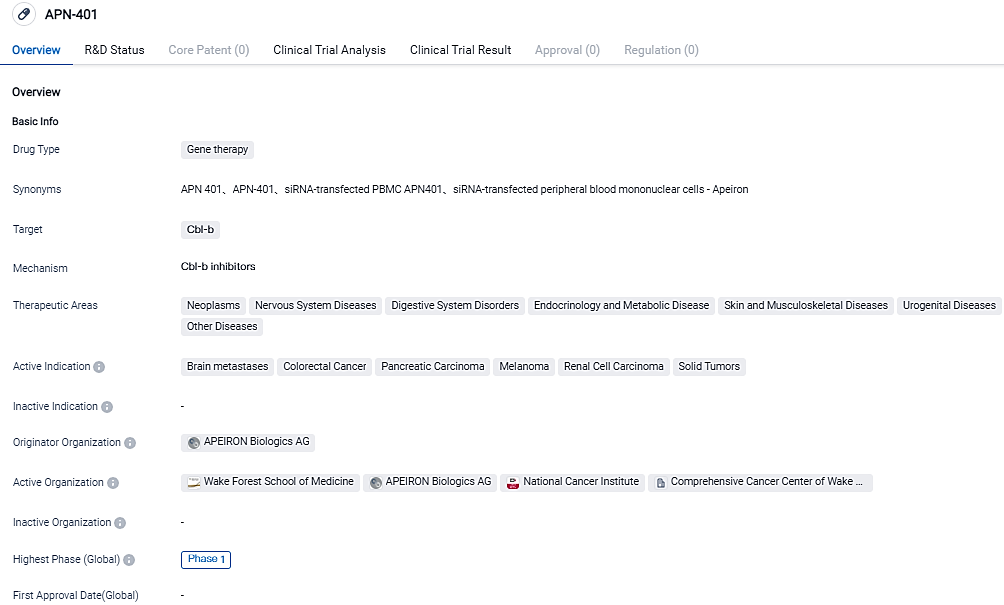
InvIOs starts a new clinical trial of innovative cell therapy APN401 for solid tumors
InvIOs GmbH, a private biotech firm advancing unique cancer treatments, revealed today that it has launched patient enrollment for a Phase 1b trial of its innovative autologous cell therapy, APN401, focusing on solid tumor patients. Subsequently, the firm has also successfully obtained a grant fund. The objective of the PALINDROM study is to identify the most effective dose of APN401 implemented in a multi-location scenario for a future Phase 2 study.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
PALINDROM is a multiple-center clinical trial that is currently being conducted in Austria. The trial is examining different dosage levels of APN401 infusions in 12 patients spread across four distinct clinical settings. The priority of the trial is to gauge the treatment’s acceptability, safety and clinical efficacy in handling patients suffering from advanced and metastatic solid tumors who haven't found established standard therapies effective or suitable.
Peter Llewellyn-Davies, Chief Executive Officer of invIOs expressed his pleasure over the Austrian government's financial aid for their latest experimental trial. "This assistance from the Austrian Life Sciences Programme is a monumental progress for Austrian biotechnological sector and especially for invIOs, as we are consistently advancing with our triad of EPiC-based projects."
Dr. Romana Gugenberger, invIO’s Chief Medical and Scientific Officer, further elaborated that their team has already showcased the safety and practical viability of their EPiC cell therapy platform technology that supports APN401 in early-phase human clinical trials. The aim behind this new research is to illustrate that EPiC is effective even when multiple production and treatment facilities are functioning.
The primary target of the PALINDROM research is to describe and validate the safety outline of APN401. Secondary objectives include parameters of immunological and anti-tumor activity, including total response rate and progression-free survival, along with the examination of pertinent biomarkers. By determining the highest tolerable and/or manufacturable dose for APN401, PALINDROM aims to lay the groundwork for an expansive Phase 2 trial of APN401.
invIOs reported encouraging clinical results from a Phase 1 trial indicating APN401's clinical activity in two intensely treated patients, one with metastatic appendix cancer, and the other with squamous cell cancer of the head and neck. This study further validated that the EPiC platform provides a strategy for swift local production of a Cbl-b silenced cell therapy, enabling outpatient treatment for patients with advanced solid tumors within a vein-to-vein time of less than 24 hours.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
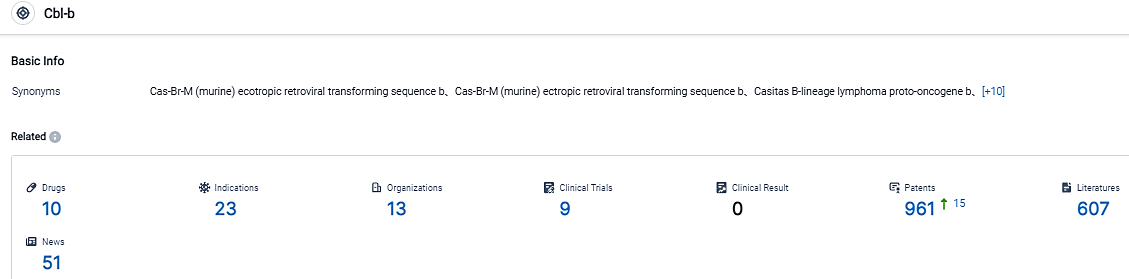 According to the data provided by the Synapse Database, As of September 6, 2023, there are 10 investigational drugs for the Cbl-b target, including 23 applicable indications, 13 R&D institutions involved, with related clinical trials reaching 9, and as many as 961 patents.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 10 investigational drugs for the Cbl-b target, including 23 applicable indications, 13 R&D institutions involved, with related clinical trials reaching 9, and as many as 961 patents.
APN401 is a novel methodology that employs invIOs' exclusive EPiC cell therapy platform to introduce siRNA into autologous peripheral blood mononuclear cells, thus silencing Cbl-b, a protein class crucial to immune cell functioning and known for its role in suppressing immune reactions to cancer.