Development of CCR8 Antibodies as a Hot Target for Immuno-Oncology Therapy in Solid Tumors
In December 2023, the Genentech team published a research paper entitled "Structural basis of antibody inhibition and chemokine activation of the human CC chemokine receptor 8" in the journal Nature Communications, which reported the structural mechanisms by which the CCR8 receptor is activated by the natural chemokine CCL1 and inhibited by antagonistic antibodies.

The C-C chemokine receptor 8 (CCR8) is a Class A G protein-coupled receptor that is highly enriched and selectively expressed in the regulatory T cells (Treg cells) within tumors. These Treg cells play a role in suppressing anti-tumor effector T cell responses. Numerous studies indicate that patients with higher levels of Treg cells in their bodies have poorer clinical outcomes and prognoses in a variety of cancers. Therefore, it is speculated that by selectively depleting Treg cells within tumors, it might be possible to reactivate anti-tumor immune responses and enhance the efficacy of cancer immunotherapies.
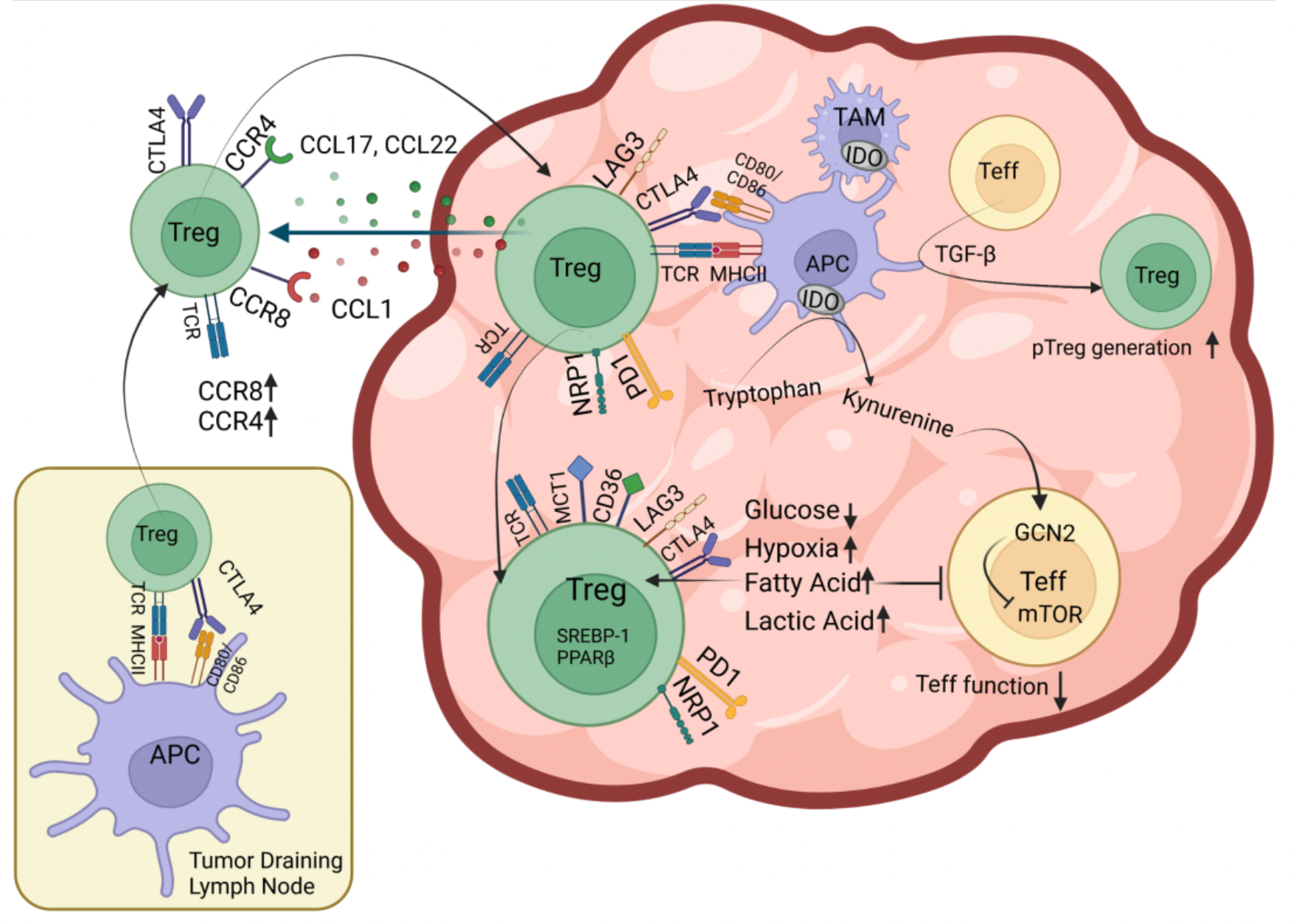
Researchers have developed an antagonistic monoclonal antibody, mAb1, that specifically binds to the extracellular region of human CCR8. Initially, New Zealand White rabbits were immunized with recombinant human CCR8, rabbi cell lines expressing human CCR8, exosomes containing CCR8, and sulfated and non-sulfated peptides from the N-terminal region of human CCR8. Single B cells were isolated using the method previously published by the Genentech team in the journal PLoS One in 2020.

Subsequently, the B cell culture supernatant was subjected to fluorescence-activated cell sorting (FACS) to isolate IgG-positive B cells, which were distributed into individual wells. These cells were then combined with CHO cells expressing human and monkey CCR8, as well as control CHO cells, for detection and identification. Ultimately, from over 480 types of anti-CCR8 antibodies capable of binding to CHO cells expressing human or monkey CCR8, antibodies were selected based on their relative mean fluorescence intensity (MFI) on the human and monkey CCR8-expressing CHO cell lines and their sequence diversity. The selected antibodies showed less than a five-fold difference in relative MFI between human and monkey CCR8 on CHO cells, identifying five unique antibody clusters (labeled Ab1-Ab5).
Researchers then constructed humanized versions of these monoclonal antibodies, designated Abx.H1L1, and expressed and purified antibodies and Fabs in the Expi293 expression system.
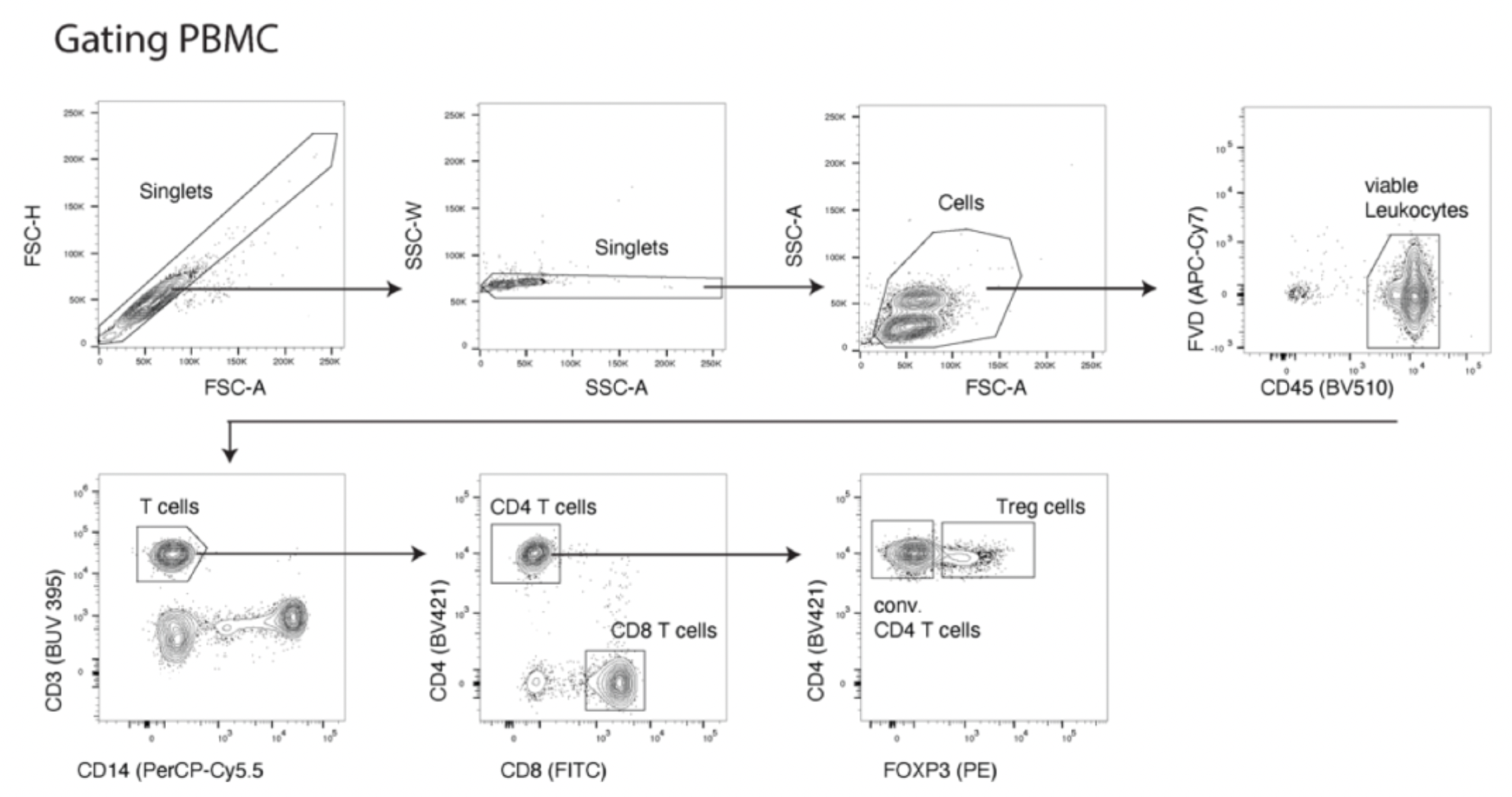
To assess the binding capability of these monoclonal antibodies to Treg cells, researchers evaluated their ability to bind to Treg cell subsets in human peripheral blood mononuclear cells (PBMCs) or dissociated tumor cells (DTCs). The experiments utilized a variety of labeled antibodies including CD45, CD3, CD8, CD4, and CD14, as well as FOXP3, to define the Treg cell population (CD45+CD14-CD3+CD8-CD4+FOXP3+). In addition, OX40 was used as a positive control, with Herceptin and anti-hIgG serving as negative controls. An example with PBMCs is shown in the figure below:
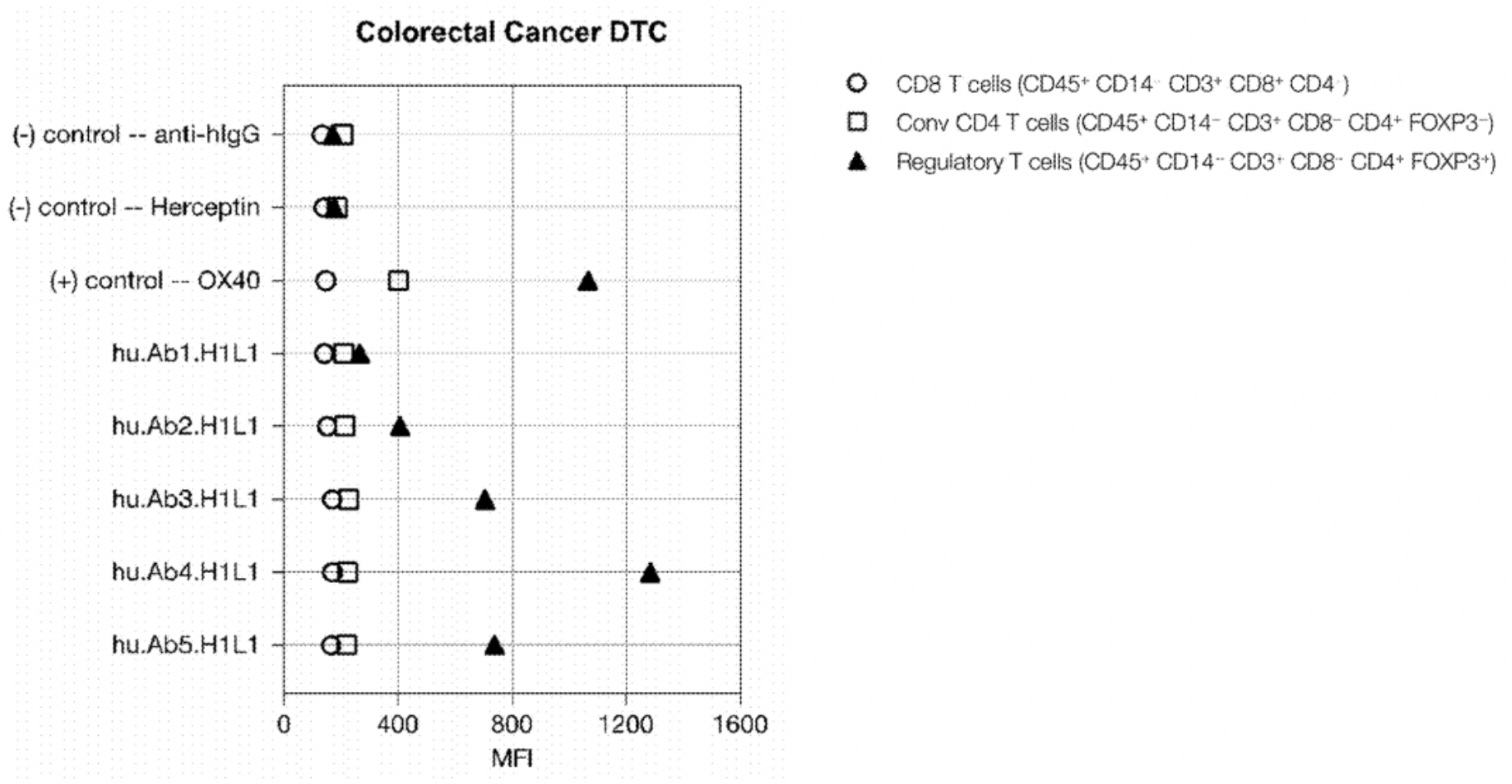
The results showed that three out of five CCR8 mAb clones could specifically stain Treg cells without staining conventional CD4 or CD8 T cells. The CCR8 relative mean fluorescence intensity was ranked as follows: hu.Ab4.H1L1 > hu.Ab5.H1L1 > hu.Ab3.H1L1.
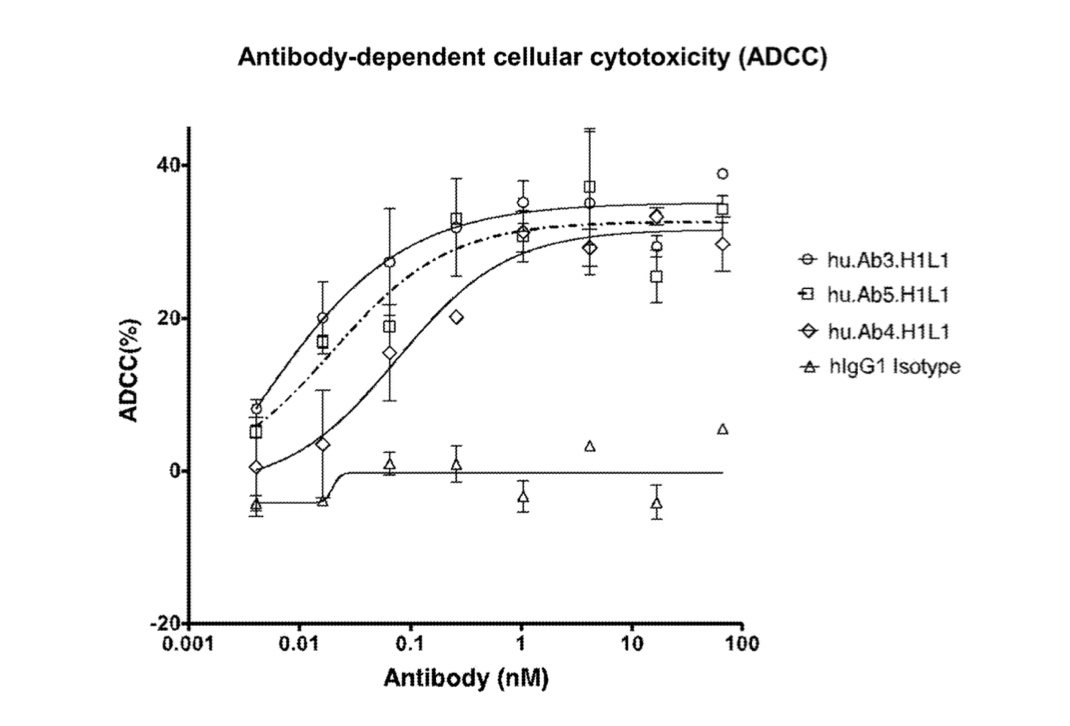
Subsequently, researchers conducted further studies on the antibody-dependent cellular cytotoxicity (ADCC) of hu.Ab3.H1L1, hu.Ab4.H1L1, and hu.Ab5.H1L1, using hIgG1 as a negative control.
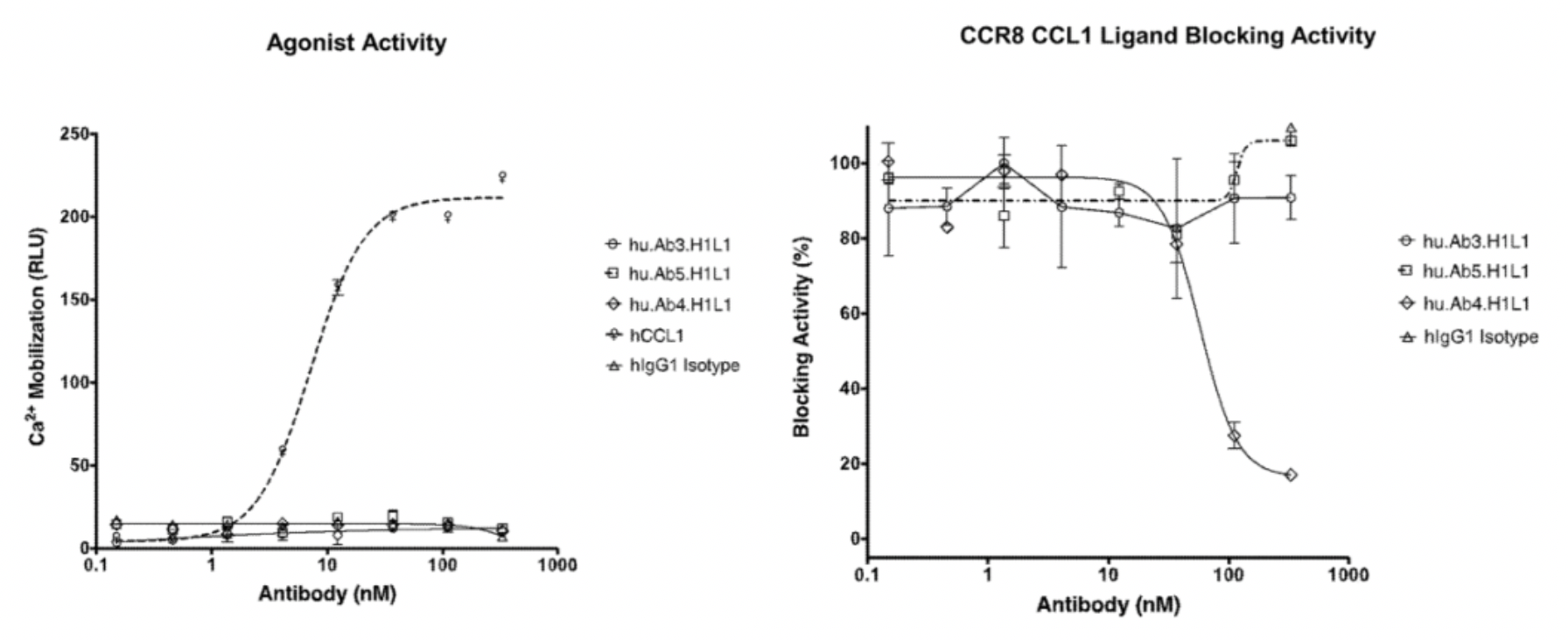
Afterward, researchers evaluated the activity of hu.Ab3.H1L1, hu.Ab4.H1L1, and hu.Ab5.H1L1 in activating or antagonizing CCR8 (using CCL1 as an agonist), with hIgG1 serving as the negative control. Downstream Ca2+ signaling changes were assessed using the FLIPR method. The results indicated that none of the three antibodies were capable of activating the downstream Ca2+ signal of CCR8, but only hu.Ab4.H1L1 was able to block the action of CCL1 on the receptor.
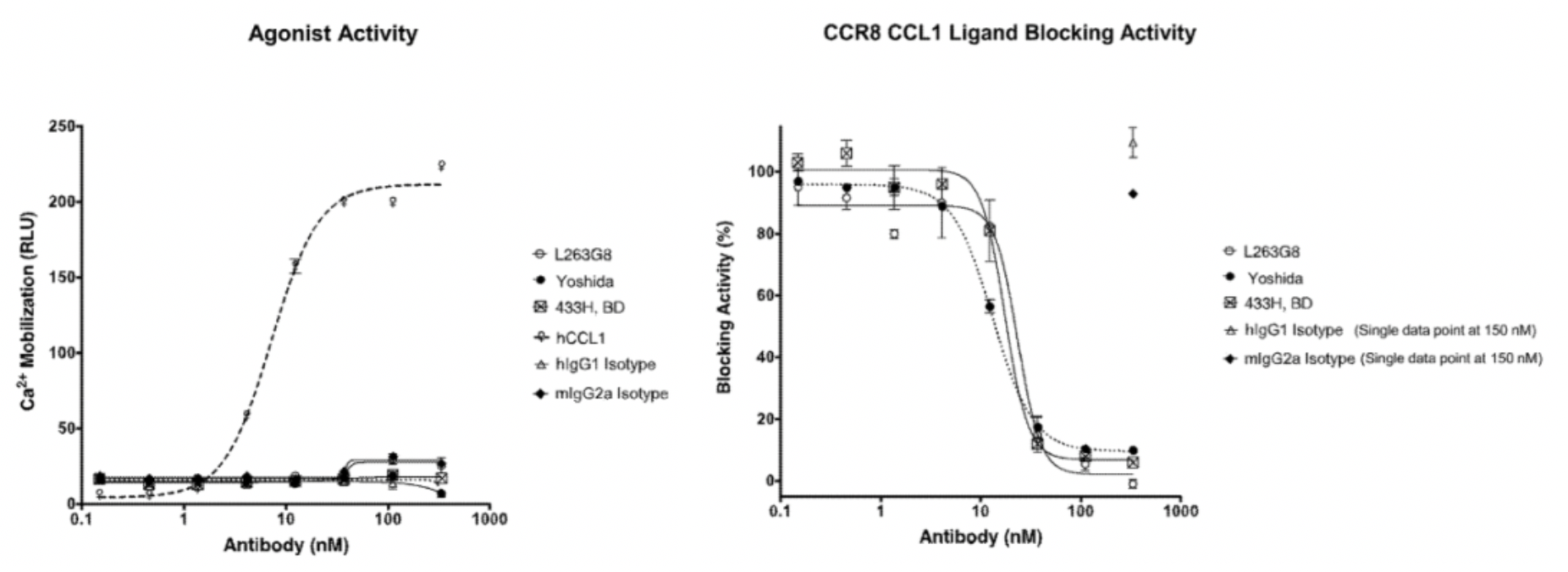
In the meantime, the author also conducted a parallel comparison of three reported antibodies against CCR8, including Yoshida (a humanized anti-human CCR8 antibody), 433H (a mouse anti-human CCR8 mAb from BD Biosciences), and L263G8 (a mouse anti-human CCR8 mAb from BioLegend). All three control antibodies were capable of antagonizing the effects of CCL1.
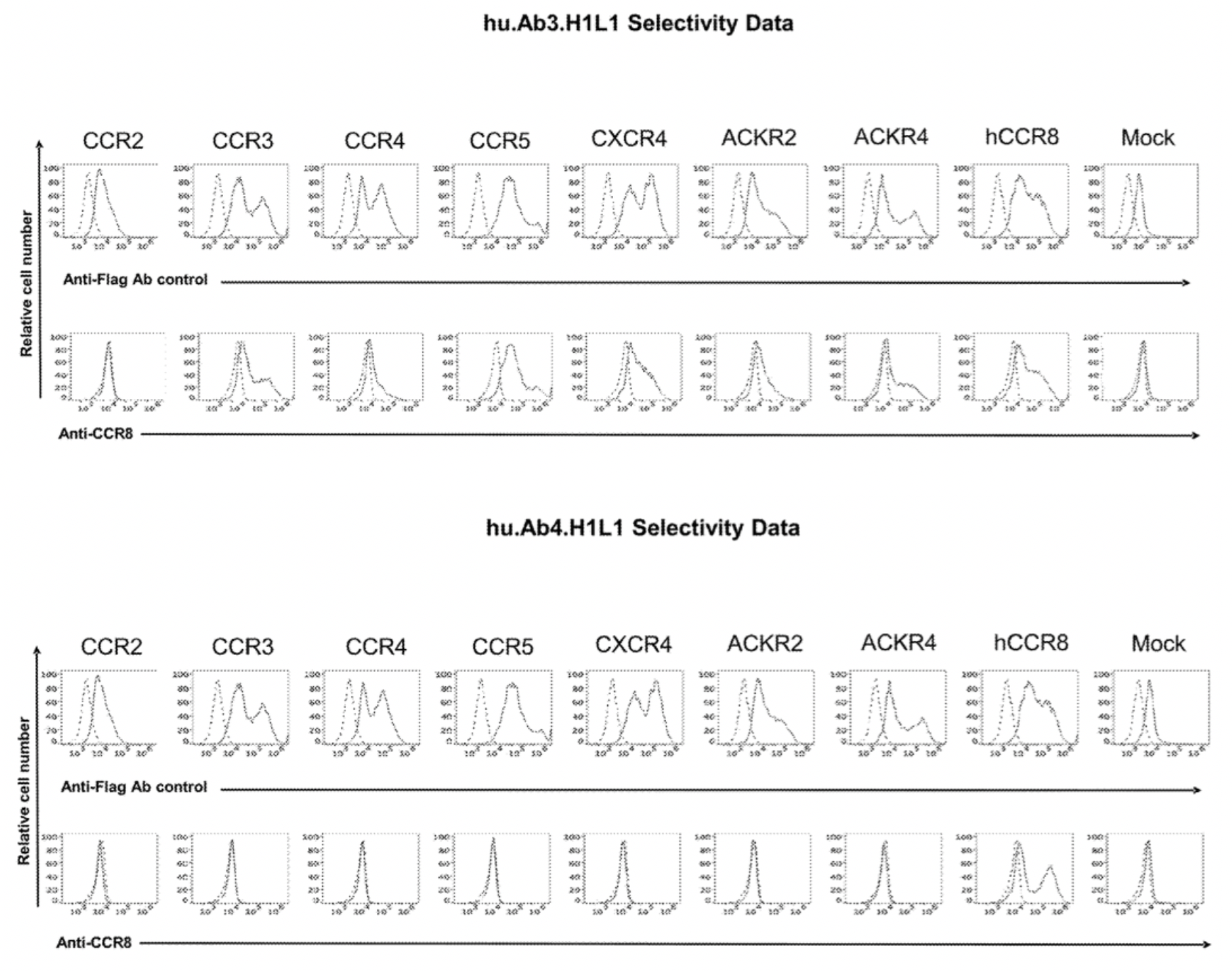
To confirm the selectivity of multiple antibodies for CCR8, the researchers used flow cytometry to identify the specific binding of hu.Ab3.HILI, hu.Ab4.HILI, and hu.Ab5.HILI, as well as Yoshida, L263G8, and 433H, to various chemokine receptors. The results showed that only the antibodies hu.Ab4.HILI and hu.Ab5.HILI stained cells expressing human CCR8, confirming their specificity for human CCR8. In contrast, the antibody hu.Ab3.HILI exhibited staining for several other GPCRs, indicating a lack of specificity.
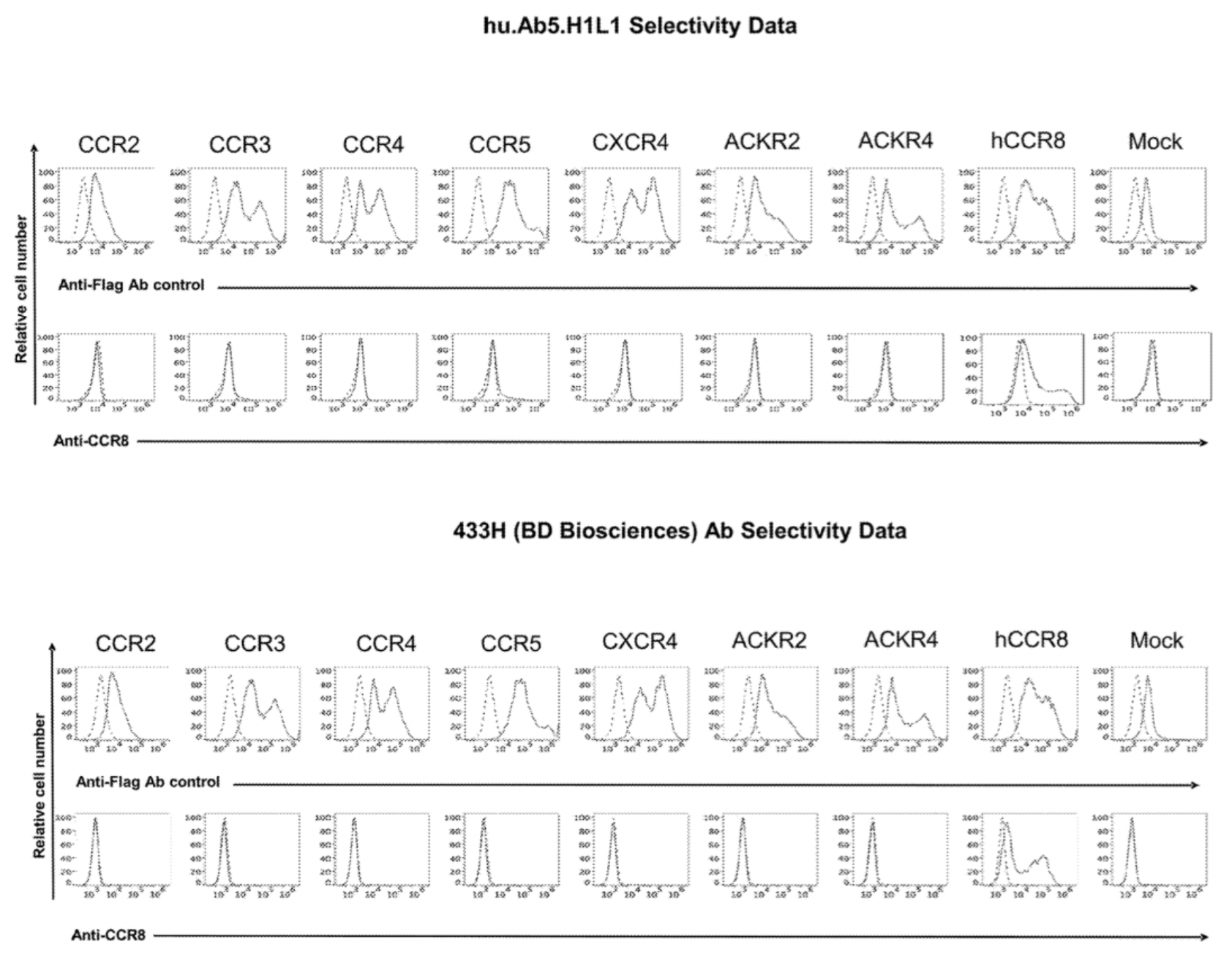
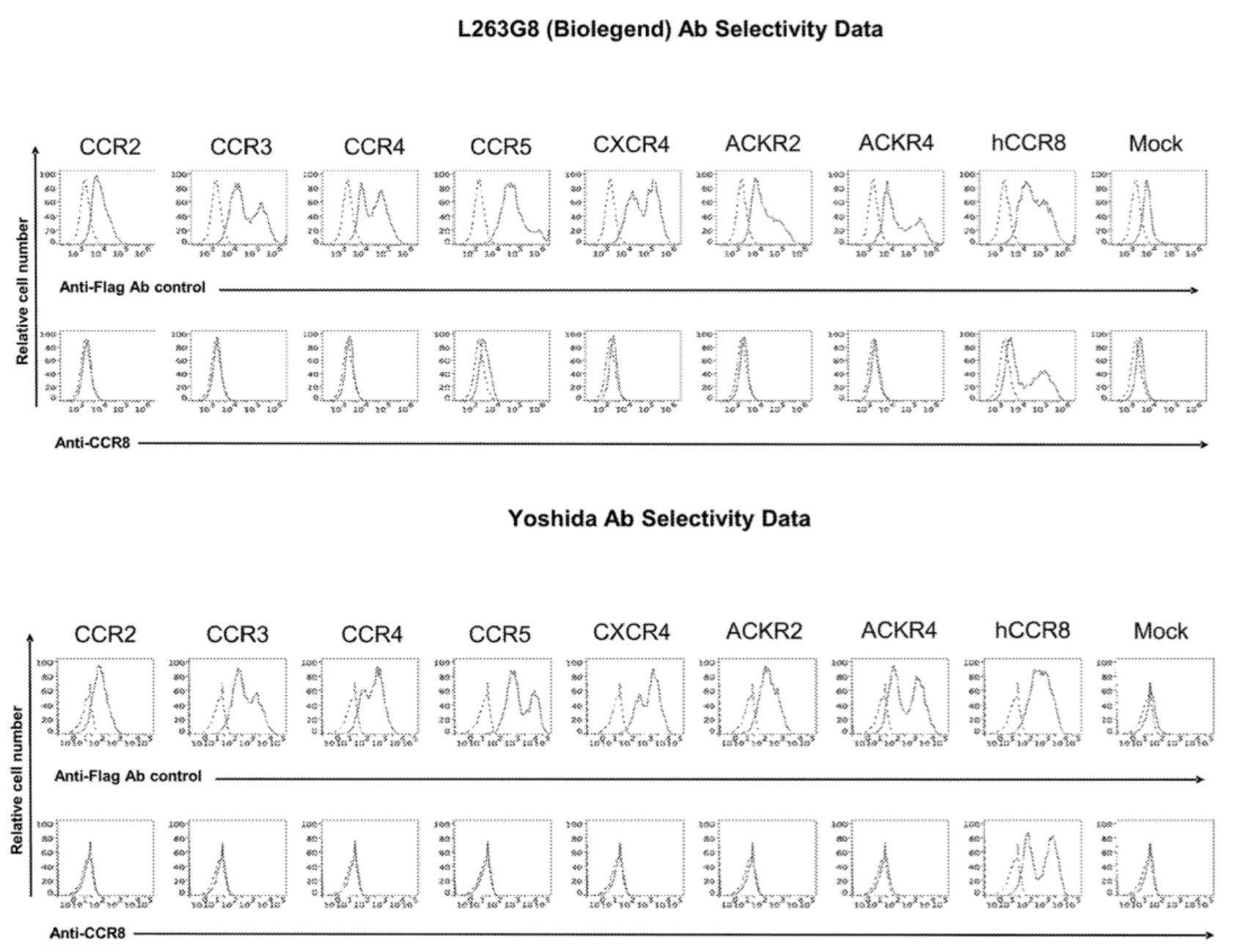
The antibody-related data mentioned above are derived from the Genentech company's patent US20230049152A1.
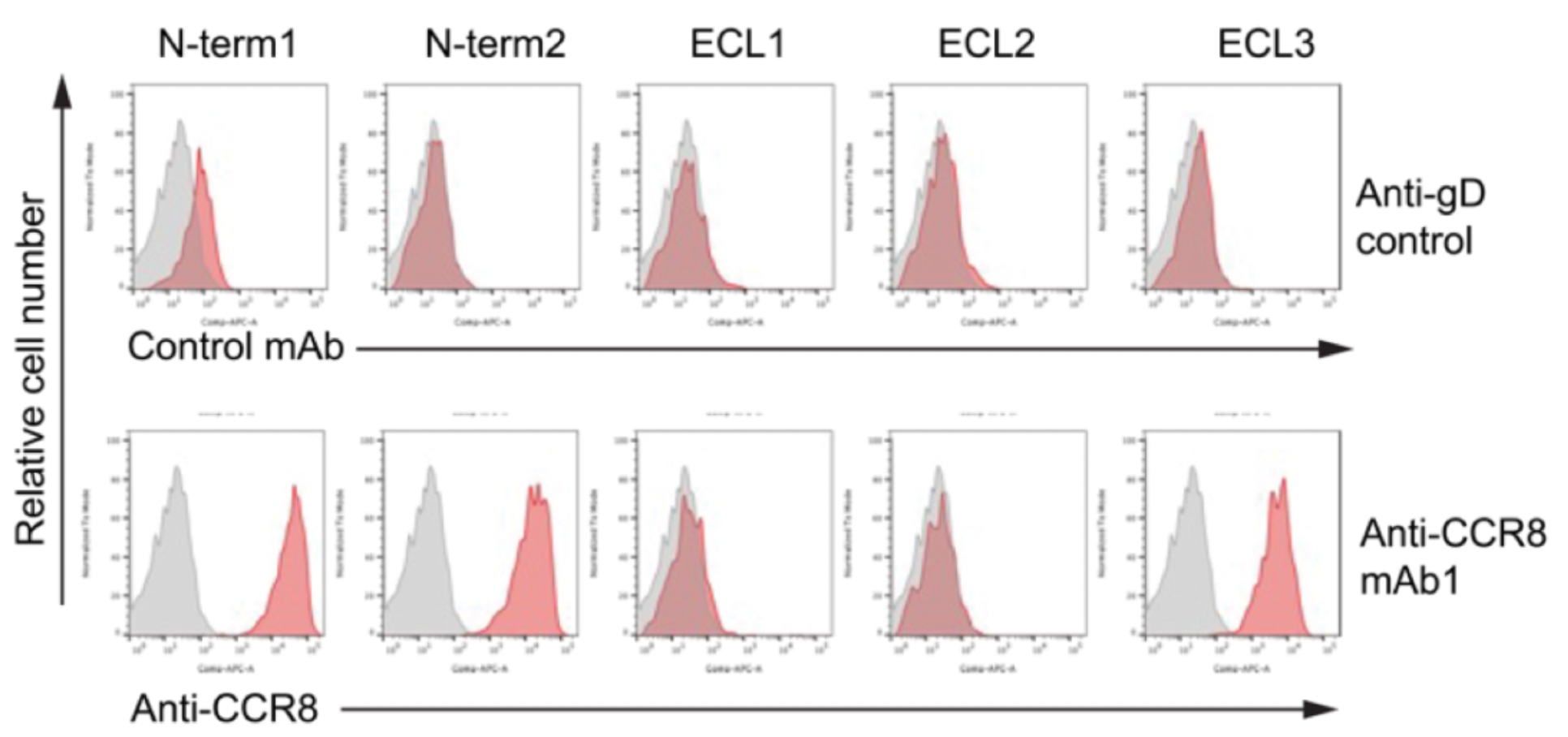
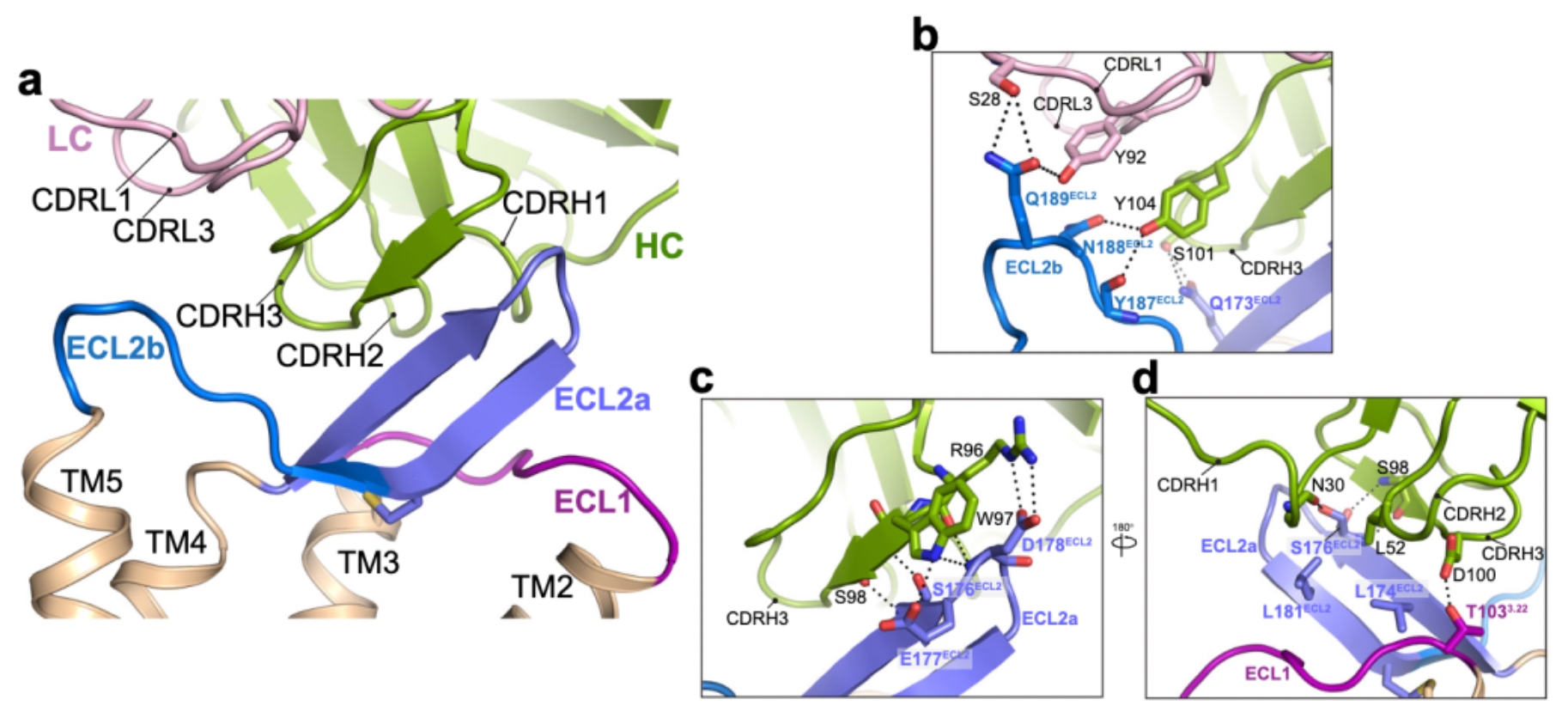
Researchers utilized chimeras of CCR8 and CCR5 to study the epitope characteristics for antibody recognition of various extracellular regions of the receptors, including N-term, ECL1, ECL2, and ECL3. The results indicate that ECL1 and ECL2 are involved in the formation of the interactive epitopes between the antibody and the receptor.
In subsequent studies, researchers employed cryo-electron microscopy to resolve the structures of the antibody mAb1 in complex with the inactive state of CCR8, as well as the structure of the active state complex of CCL1 with CCR8 and the downstream Gi subtype protein.
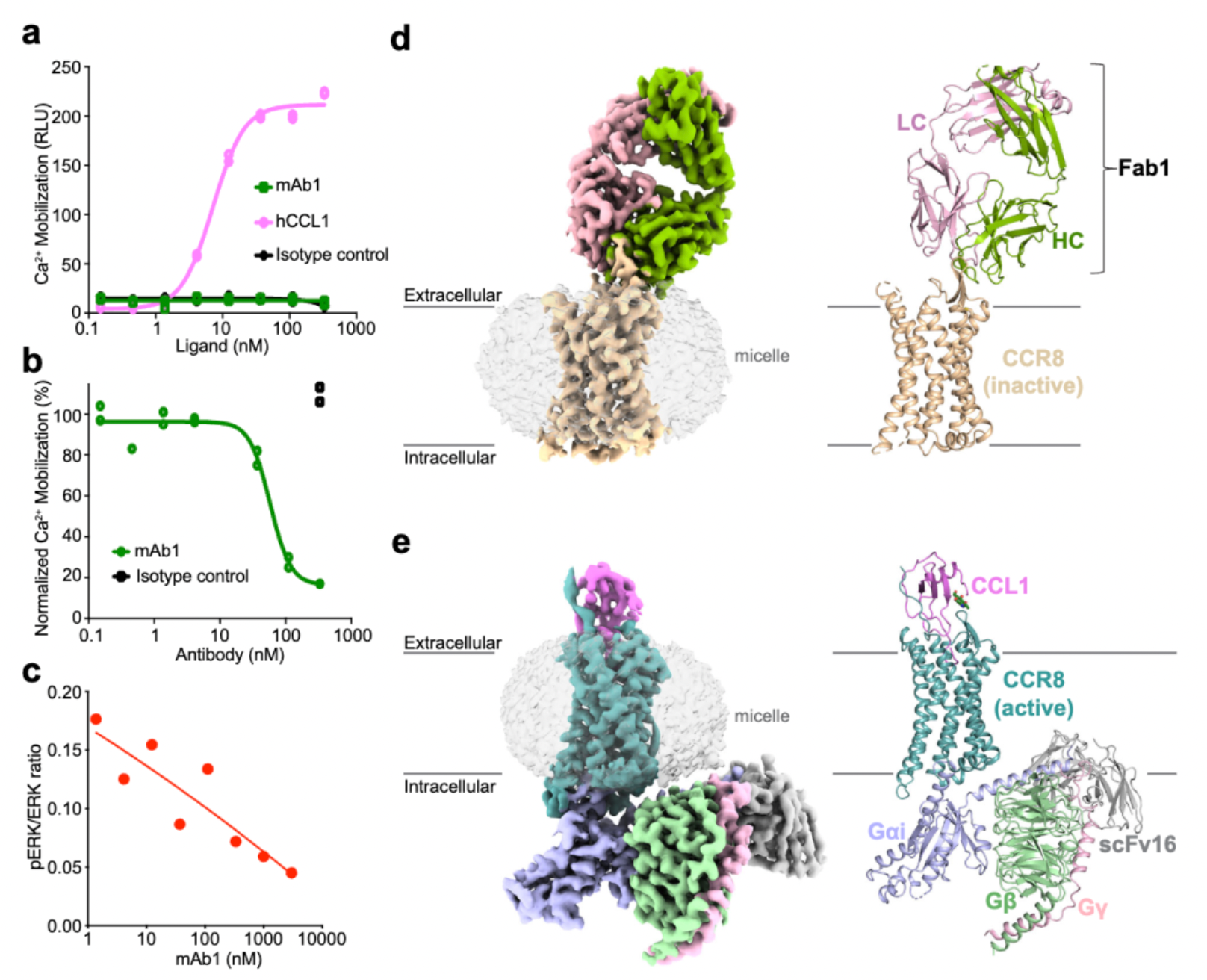
Cryo-EM structural analysis confirms that the primary interaction regions between the antibody fragment Fab1 and the receptor are ECL1 and ECL2. Here, Fab1 forms an extensive interaction interface with CCR8's ECL1 and ECL2, primarily driven by electrostatic interactions, which is predominantly mediated by its CDRH3, with contributions from CDRH1, CDRH2, CDRL1, and CDRL3.
References:
1.Structural basis of antibody inhibition and chemokine activation of the human CC chemokine receptor 8
2.https://www-nature-com.libproxy1.nus.edu.sg/articles/s41467-023-43601-8