Takeda's Phase 2 Results for Anti-CD38 Antibody Mezagitamab (TAK-079) Show Promise in Immune Thrombocytopenia Treatment
Takeda has reported encouraging preliminary outcomes from a controlled, randomized, Phase 2 study that was conducted in a double-blinded manner, assessing the effects and safety profile of mezagitamab (TAK-079) when administered to patients diagnosed with primary immune thrombocytopenia that is either chronic or not responding to initial treatments. The study aimed to determine the drug’s impact on patient health and its potential side effects.
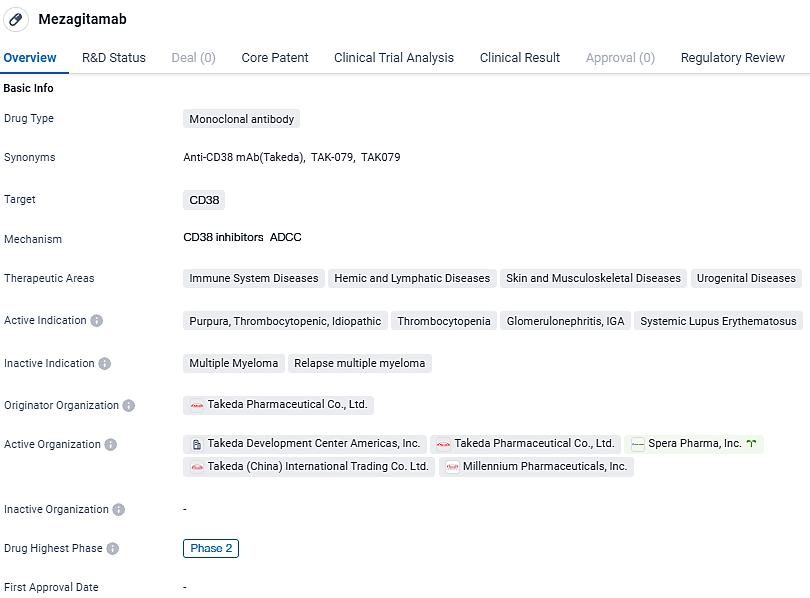
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Mezagitamab is a fully human antibody of the IgG1 subtype that selectively targets and diminishes cells expressing CD38.
In the clinical study TAK-079-1004, researchers investigated the effects of three dosage levels of mezagitamab administered subcutaneously compared to a placebo over an eight-week period on individuals with either chronic or non-resolving primary Immune Thrombocytopenia (ITP). Early results from this Phase 2 research provided encouraging data regarding the treatment's safety and its potential benefits.
Throughout all patient groups involved in the trial, mezagitamab was shown to be safe and well-accepted. Compared to the placebo group, the tested doses of mezagitamab all yielded a more significant increase in platelet counts. These rises correlated with dosage, with the top dose delivering the most substantial increase in platelets. Patients undergoing treatment with mezagitamab experienced quick elevation in platelet levels, and this effect extended beyond the period of active treatment.
Chinwe Ukomadu, Takeda's GI & Inflammation Therapeutic Area Unit leader, emphasized the strong therapeutic potential of mezagitamab, highlighted by the Phase 2 findings. Ukomadu pointed out that many ITP patients still face challenges with current treatments, either due to insufficient responses or difficulties in managing side effects and risks of disease recurrence. For such individuals, mezagitamab could represent a pivotal shift toward effective disease management and even remission.
The goal of using mezagitamab for therapy is to swiftly and stably elevate platelet levels, usually restoring them to normal counts quickly. The U.S. FDA has awarded Mezagitamab Orphan Drug Status for its role in ITP therapy, and the program has also attained Fast Track Designation to facilitate its development and potential approval.
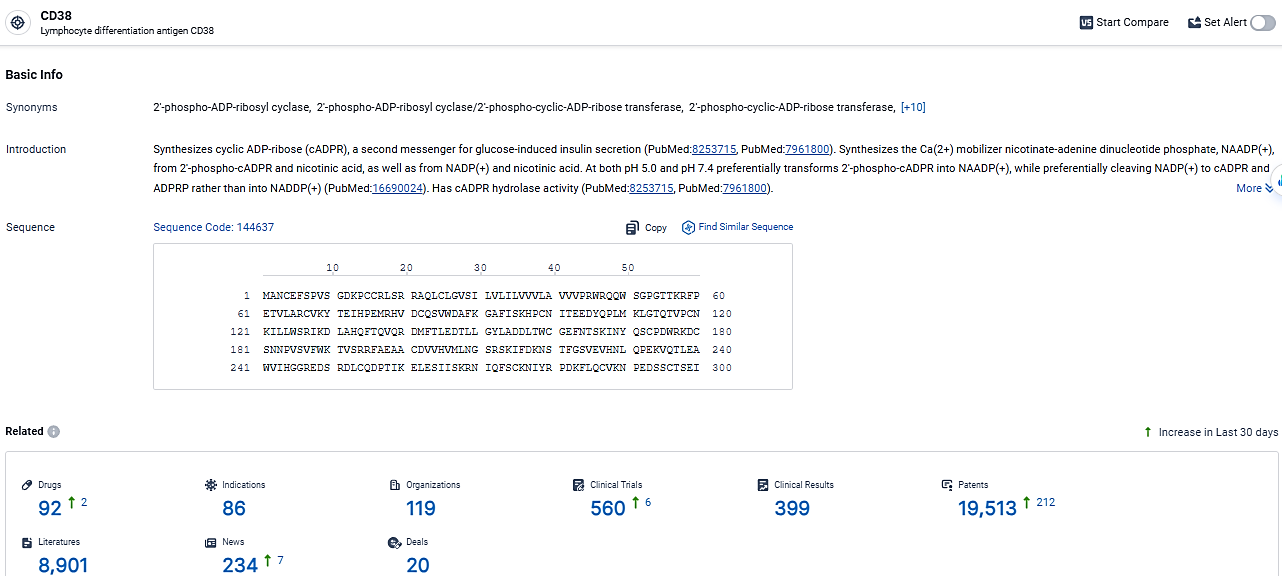
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 14, 2024, there are 92 investigational drugs for the CD38 target, including 86 indications, 119 R&D institutions involved, with related clinical trials reaching 560, and as many as 19513 patents.
Mezagitamab targets CD38 and has shown potential in treating various immune system, hemic and lymphatic system, skin and musculoskeletal system, and urogenital system diseases. With its orphan drug status and current Phase 2 trials, Mezagitamab represents a promising candidate for future therapeutic options in these areas.