Distinct Mechanisms and Therapeutic Potential of ASOs and siRNAs in Precision Medicine
In the field of modern gene therapy, antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) are two critical types of small nucleic acid drugs that are opening new avenues for treating genetic and other complex diseases. Although both aim to achieve therapeutic effects by regulating gene expression, they differ significantly in their sites of action and delivery mechanisms. This article explores the unique characteristics of these two technologies and their respective advantages in clinical applications, revealing how they play indispensable roles in the era of precision medicine.
With the continuous advancement of biotechnology, ASOs and siRNAs have emerged as star molecules in gene-silencing therapies. However, their intrinsic properties and applicable scenarios differ fundamentally—ASOs can regulate gene expression both inside and outside the cell nucleus, while siRNAs primarily exert their effects in the cytoplasm. These differences are not only reflected in their biological mechanisms but also in the diversity of their delivery technologies and therapeutic applications. Patsnap Bio not only supports the efficiency of scientific research but also provides valuable insights for drug developers, helping them secure advantageous positions in the competitive market. Next, we will delve into the working mechanisms of these two types of small nucleic acid drugs and their potential applications in treating various diseases.
ASO Drugs Act in the Cell Nucleus
ASOs can be designed to target RNA both inside and outside the cell nucleus, including precursor mRNA (pre-mRNA) and mature mRNA, thereby regulating gene expression at the post-transcriptional level. By directly binding to target RNA, ASOs can induce RNA degradation (e.g., RNase H-mediated degradation), alter splicing patterns to exclude critical exons, or inhibit protein synthesis by blocking the binding of translation initiation factors to mRNA. Since RNase H is active in both the nucleus and the cytoplasm, ASOs leveraging this mechanism can affect transcripts such as immature pre-mRNA and long non-coding RNA (lncRNA) within the nucleus, indirectly modulating protein synthesis.
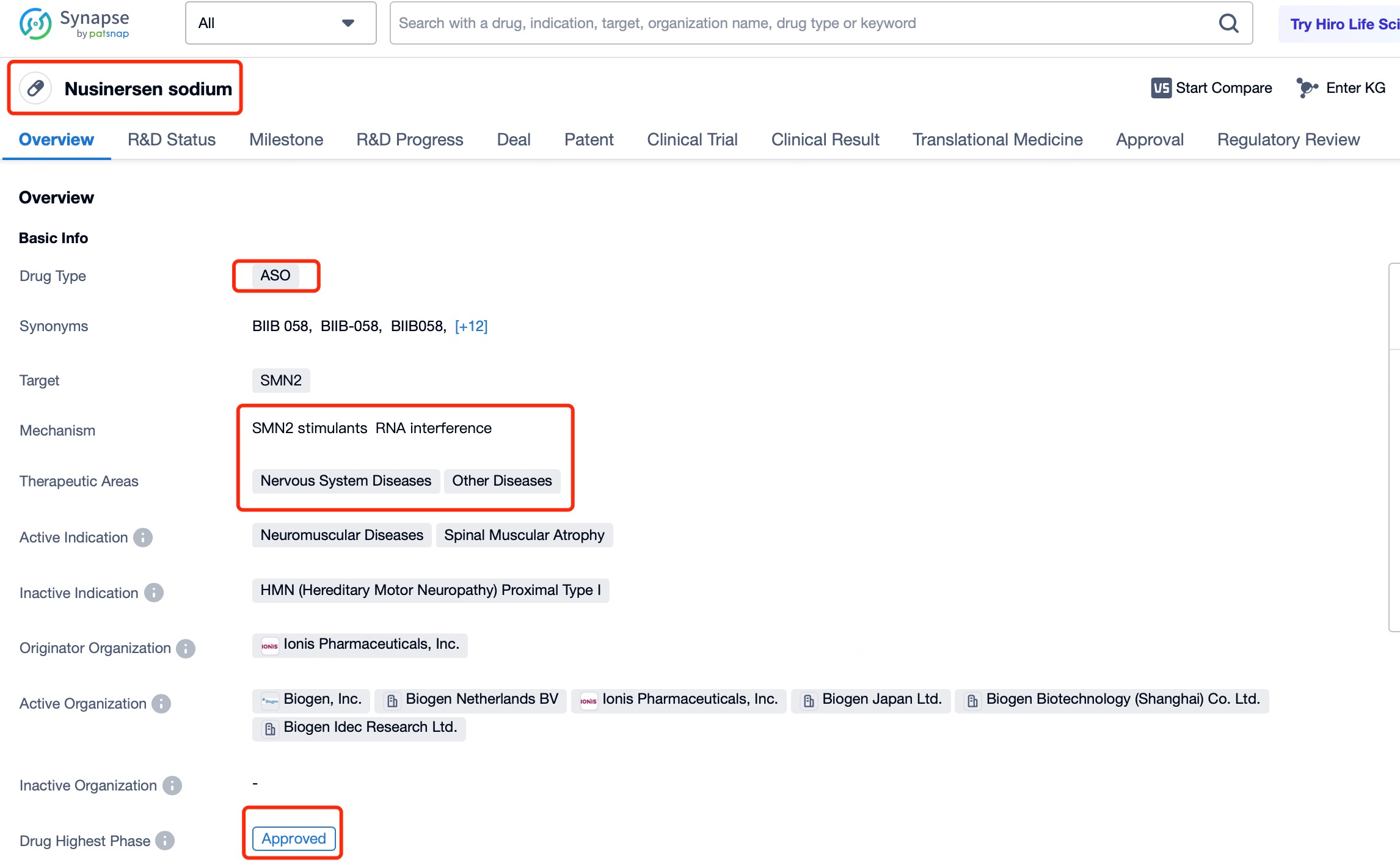
A notable example is Nusinersen (brand name Spinraza), a drug for treating spinal muscular atrophy (SMA). SMA is a genetic disorder caused by mutations in the SMN1 gene, leading to a deficiency of survival motor neuron (SMN) protein, resulting in motor neuron death and muscle weakness. With tools like Patsnap Bio and Patsnap Synapse, detailed information about Nusinersen and other ASOs can be accessed quickly, including their mechanisms of action, delivery methods, and clinical trial data. Nusinersen is delivered to the central nervous system via intrathecal injection, directly targeting neuronal cells in the spinal cord. By entering the nucleus and influencing pre-mRNA splicing, Nusinersen demonstrates the ability of ASOs to regulate gene expression within the nucleus. This mechanism not only overcomes the limitations of traditional therapies but also provides an effective treatment option for SMA patients.
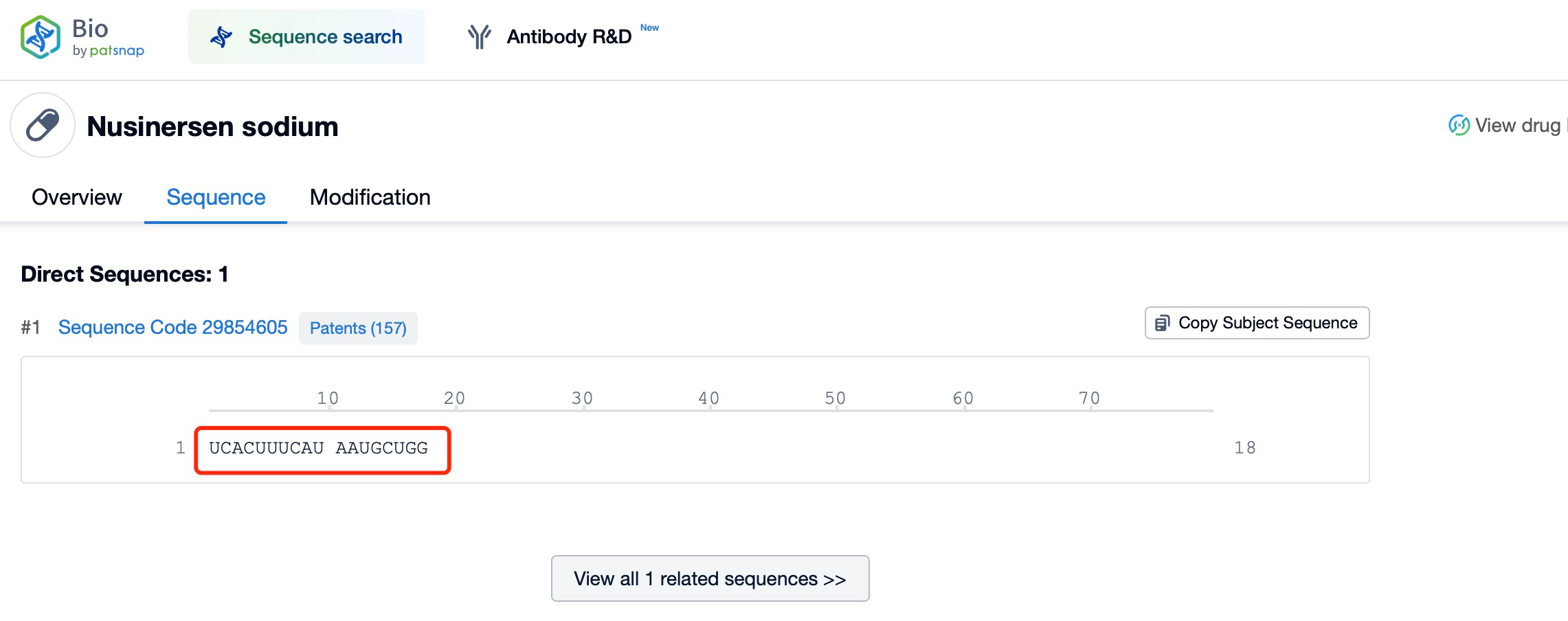
siRNA drugs primarily exert their effects in the cytoplasm
By binding to the RNA-induced silencing complex (RISC), particularly to the Argonaute 2 (Ago2) protein, siRNA utilizes its endonuclease activity to cleave specific mRNA sequences complementary to the siRNA, thereby silencing the target gene's expression. This mechanism leads to mRNA cleavage and subsequent degradation, reducing the production of proteins encoded by these mRNAs. Because siRNAs primarily act in the cytoplasm, they target mature mRNA that has already exited the nucleus.
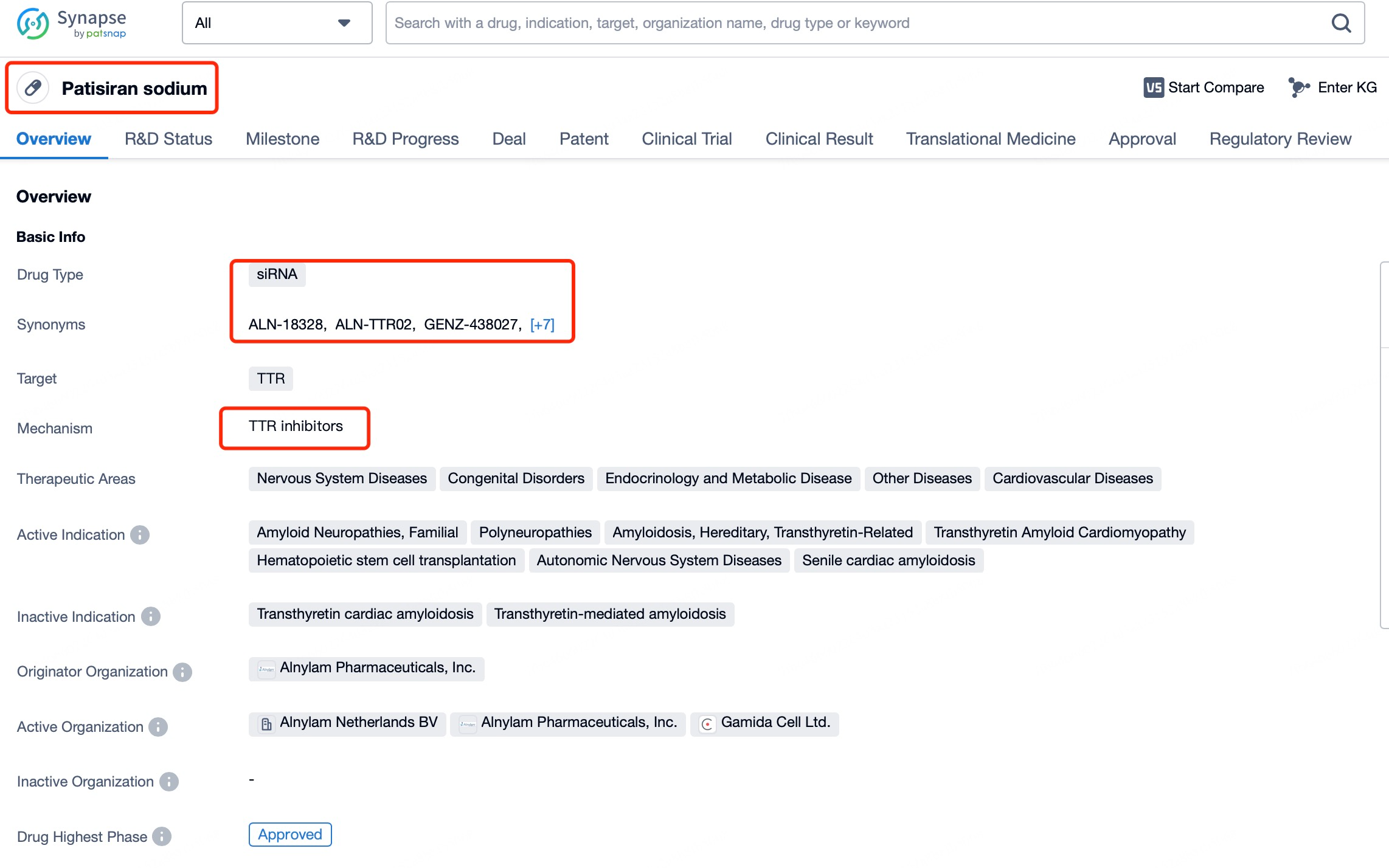
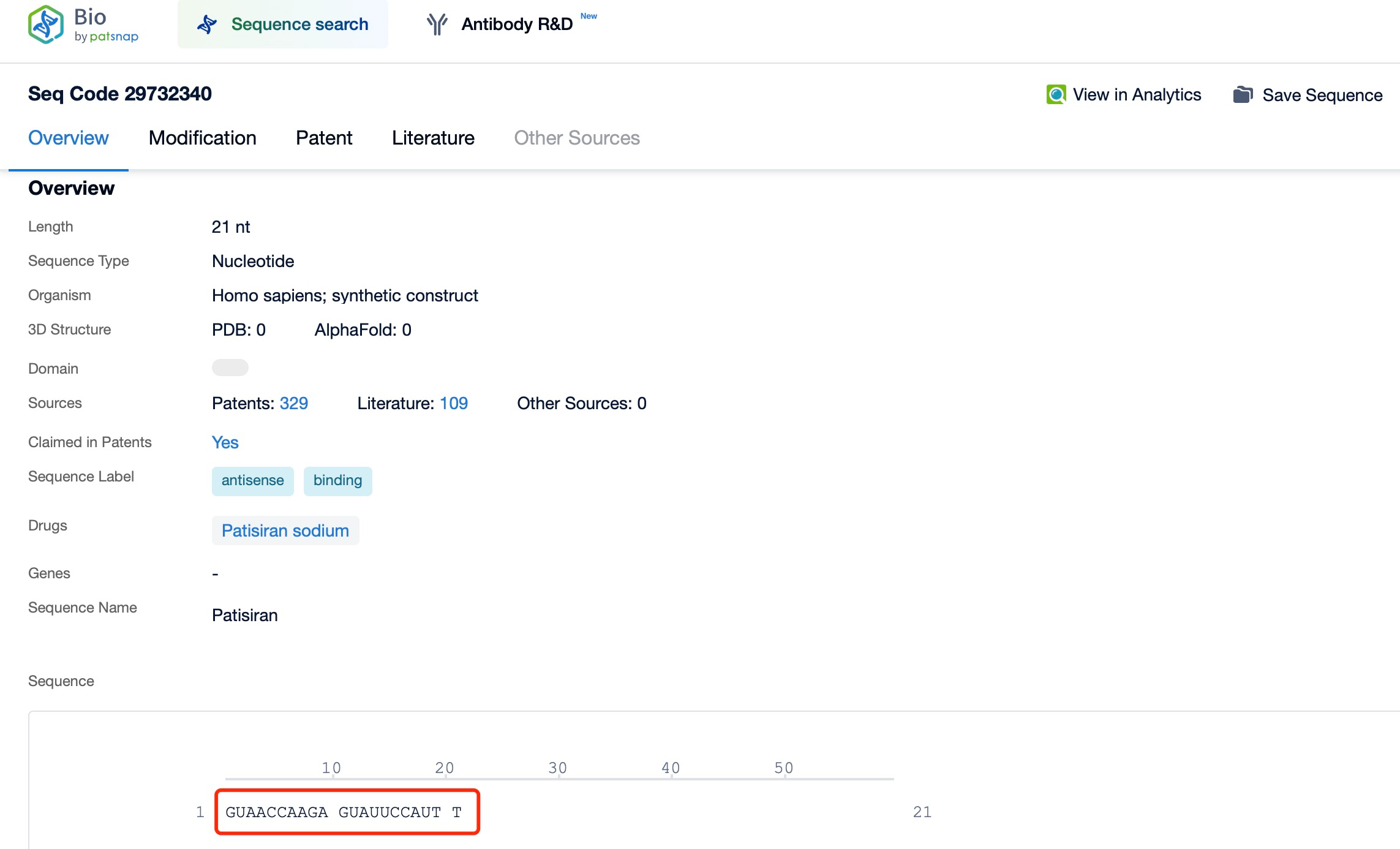
A representative example is Patisiran (brand name Onpattro), a treatment for hereditary transthyretin-mediated amyloidosis (hATTR). This disease is caused by mutations in the transthyretin (TTR) gene, leading to the deposition of abnormal TTR protein in various tissues, resulting in symptoms like neuropathy and cardiac damage. Through tools such as Patsnap Bio and Patsnap Synapse, it has been shown that Patisiran targets and degrades the mRNA encoding abnormal TTR protein, thereby reducing its production and alleviating amyloidosis symptoms. To ensure effective delivery, Patisiran employs lipid nanoparticle (LNP) technology, which protects the siRNA from nuclease degradation and facilitates its cellular uptake via endocytosis. Once in the cytoplasm, the siRNA binds to the target mRNA and triggers its degradation. Because siRNAs primarily act in the cytoplasm, they focus on mature mRNA already processed and exported from the nucleus.
ASOs exhibit a broader range of action sites
Chemically modified ASOs achieve higher stability and selectivity, enabling them to resist degradation and bind specifically to target RNA with precision. These advancements allow ASOs to reach diverse tissues, including not only easily accessible sites like the liver but also more challenging areas such as muscle tissue and the central nervous system. This broad tissue distribution capability highlights ASOs' immense potential in treating diseases associated with specific organs or tissues.
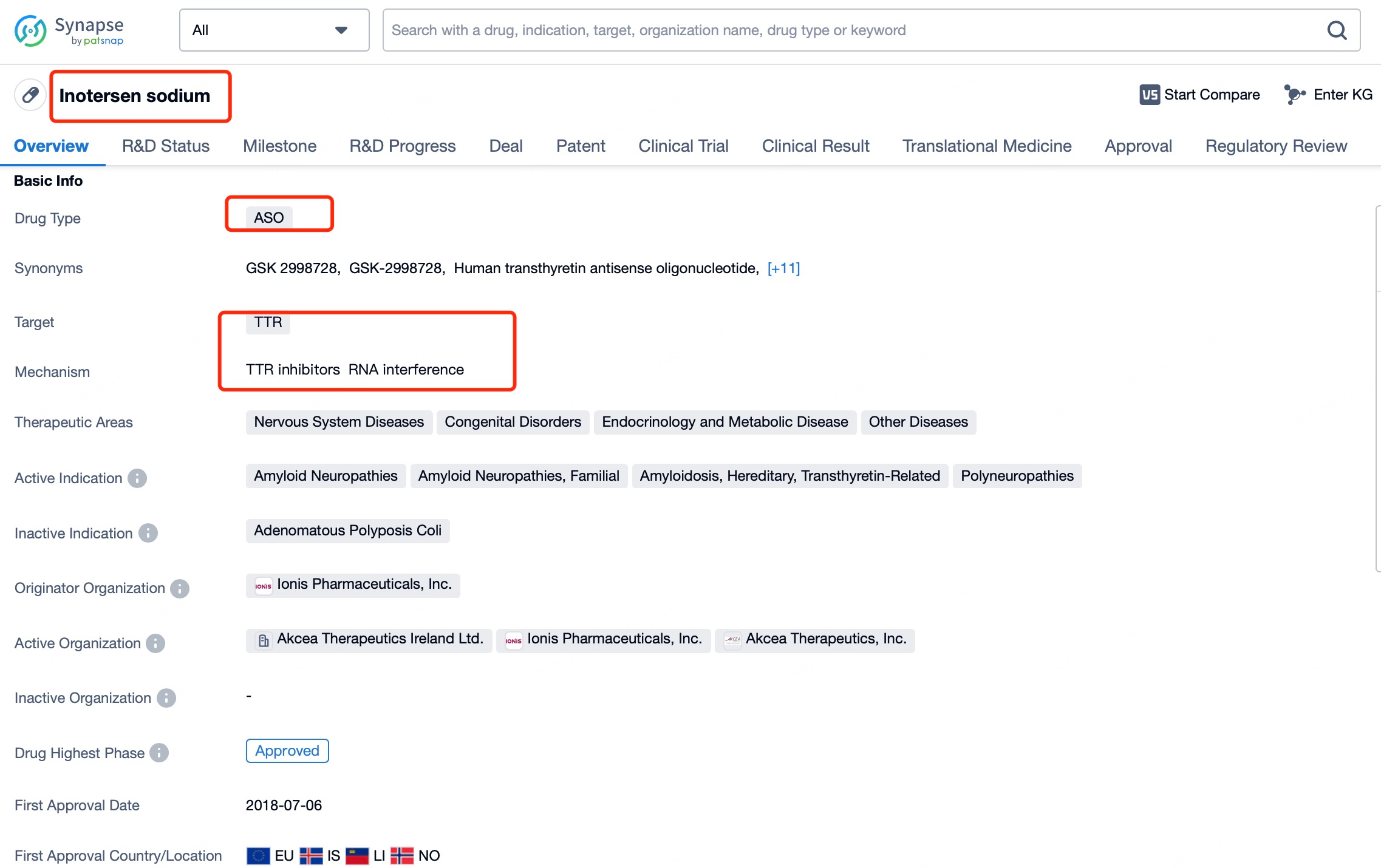
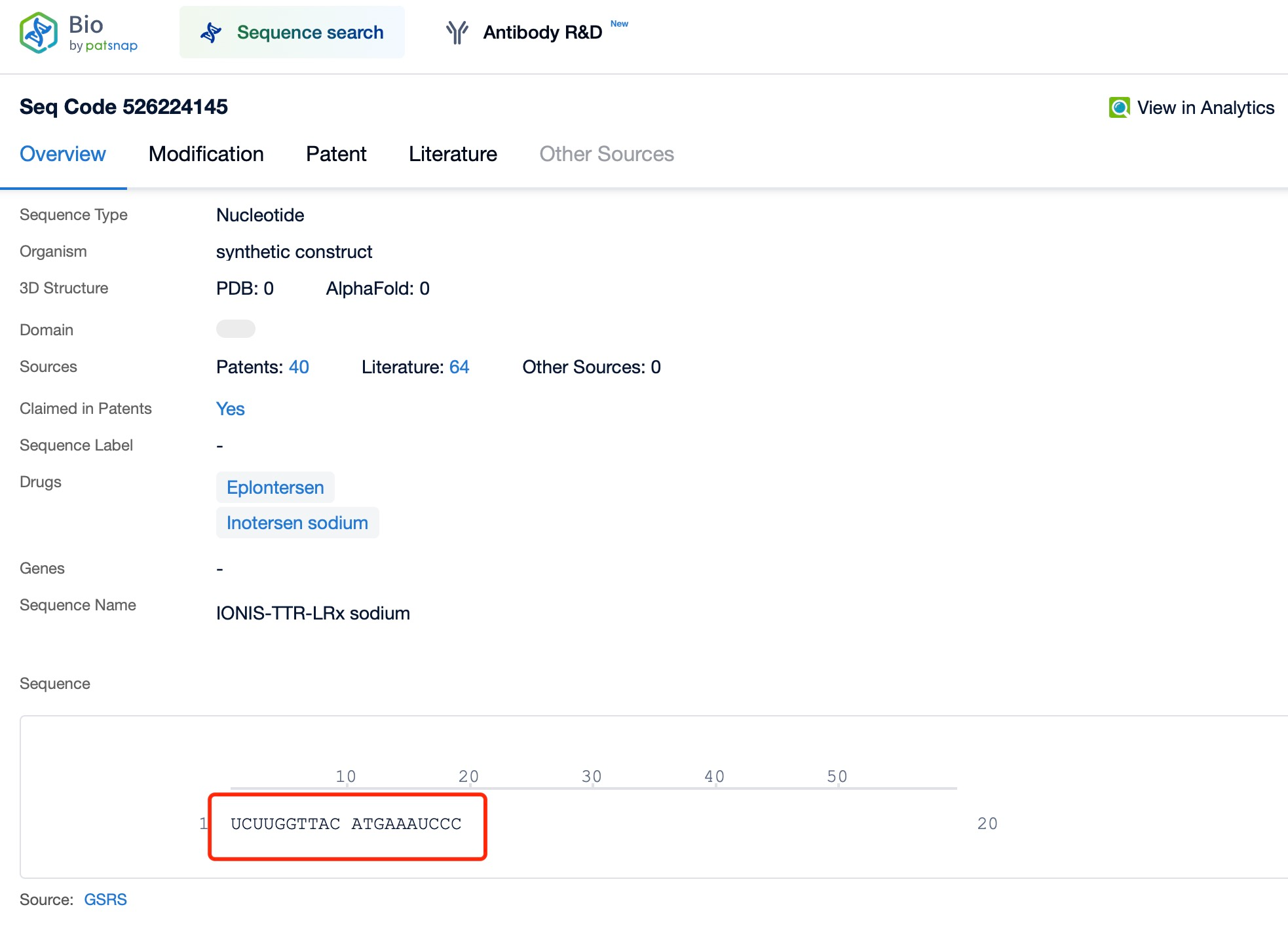
Inotersen (brand name Tegsugo) exemplifies this versatility. It is a chemically modified ASO developed for hATTR treatment. According to insights from Patsnap Bio and Patsnap Synapse, Inotersen targets the mRNA encoding TTR, inducing RNA degradation to reduce abnormal TTR protein production. Administered via subcutaneous injection, it effectively reaches various tissues to exert its therapeutic effects.
In comparison, siRNAs tend to focus on the liver. As an organ with robust absorptive and metabolic capacity, the liver is an ideal target for macromolecular drugs like siRNAs. The liver's abundance of endocytosis pathways facilitates siRNA cellular uptake, and its stable internal environment helps maintain siRNA structural integrity and functionality. Moreover, delivery systems designed with liver-specific GalNAc (N-acetylgalactosamine) ligands can selectively recognize and bind to receptors on hepatocyte surfaces, enabling efficient siRNA delivery. This targeted delivery mechanism not only enhances siRNA accumulation in the liver but also minimizes off-target effects and potential side effects. With this precise delivery strategy, siRNAs can effectively suppress target gene expression in the liver, achieving their intended therapeutic effects.
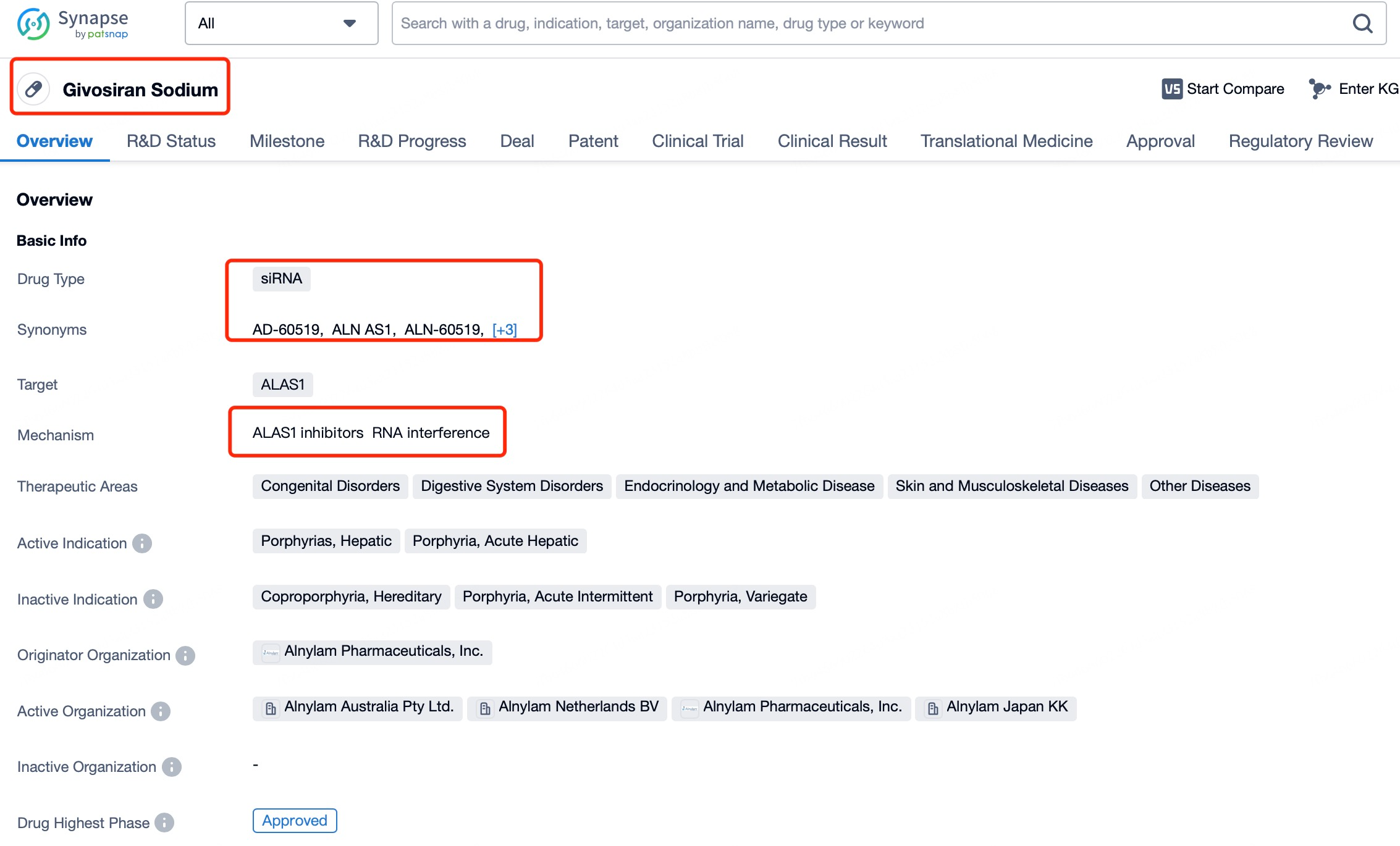
Givosiran (brand name Givlaari) is a prime example of an siRNA drug designed to treat acute hepatic porphyria (AHP), a rare genetic disorder caused by disruptions in porphyrin metabolism. Patients with AHP often experience severe pain, neuropathy, and other debilitating symptoms. Givosiran works by targeting and suppressing the expression of delta-aminolevulinic acid synthase 1 (ALAS1), thereby reducing the excessive production of porphyrin precursors and alleviating the symptoms of AHP.
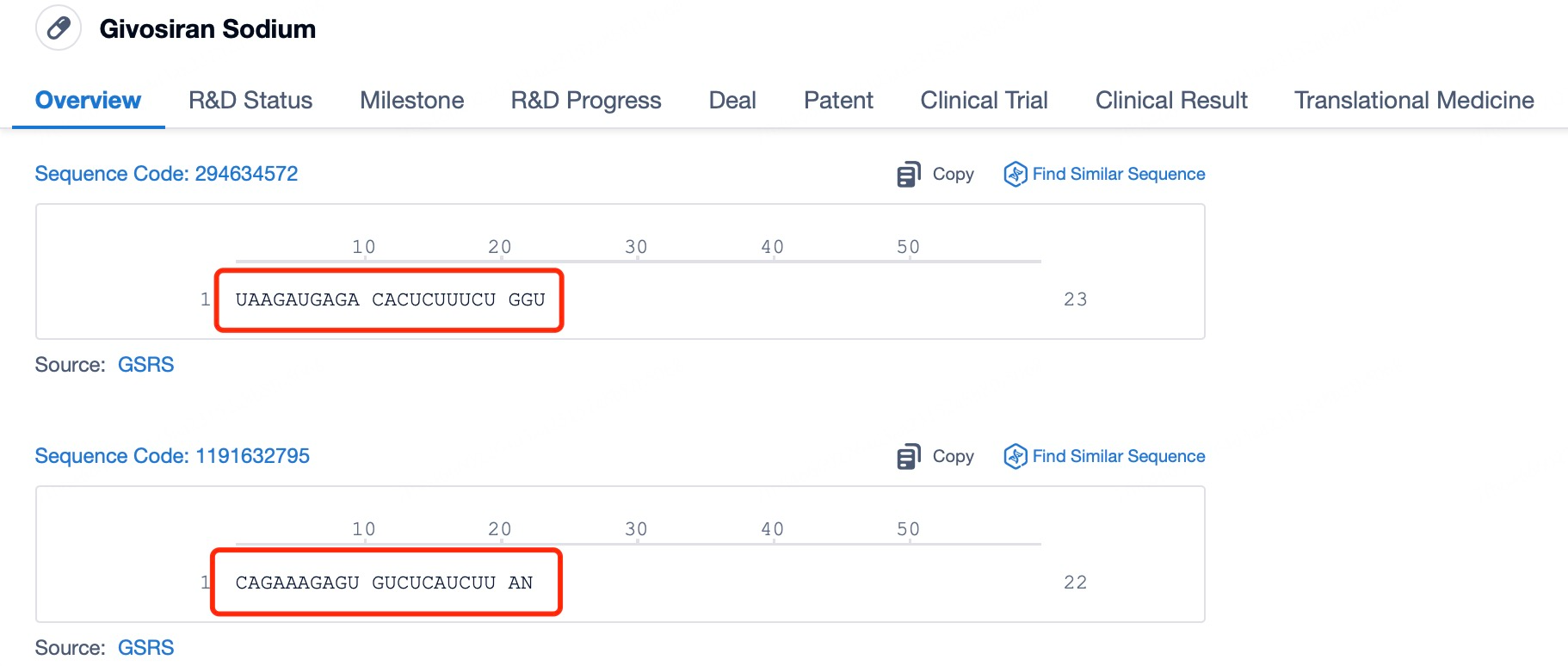
Using insights from Patsnap Bio and Patsnap Synapse, Givosiran employs a liver-specific delivery strategy incorporating GalNAc (N-acetylgalactosamine) ligands in its delivery vehicle. The liver’s robust absorption and metabolic capacity, combined with its endocytosis-rich environment, facilitate the effective cellular uptake of siRNA. Additionally, the stable environment within the liver supports the structural integrity of siRNA, ensuring its therapeutic function remains unaffected.
Despite these advances, ongoing innovations in delivery technologies, particularly antibody-oligonucleotide conjugates (AOCs) and nanoparticle systems, are gradually expanding the therapeutic reach of siRNAs beyond liver-targeted applications. These developments hold promise for siRNA treatments aimed at non-hepatic tissues, broadening the scope of potential diseases addressable by this class of drugs.
Summary
While ASOs and siRNAs both represent small nucleic acid therapies for gene expression modulation, they exhibit significant differences in their sites of action and delivery strategies. ASOs can act on both nuclear and cytoplasmic RNA, including pre-mRNA and mature mRNA, and benefit from chemical modifications that enhance stability and targeting, making them suitable for a variety of tissues and organs. This versatility underscores their potential in treating genetic diseases, particularly those requiring nuclear gene expression regulation, such as Inotersen (Tegsugo) for hATTR.
On the other hand, siRNAs primarily operate in the cytoplasm, silencing specific mRNAs through their interaction with the RNA-induced silencing complex (RISC). Their delivery often relies on lipid nanoparticle (LNP) systems, demonstrating high efficacy in liver-targeted therapies via specialized carriers like GalNAc ligands. This approach not only enhances therapeutic effectiveness but also minimizes side effects. With continuous advancements in delivery technologies, including AOCs and novel nanoparticles, siRNA therapeutics are expected to extend their applications to non-liver tissues, opening new possibilities in the field of gene therapy.
If you are interested in understanding the market dynamics and competitive landscape in ASO, click on Patsnap Synapse.
To learn more about bio sequences and modifications, click on Patsnap Bio.
Refrence
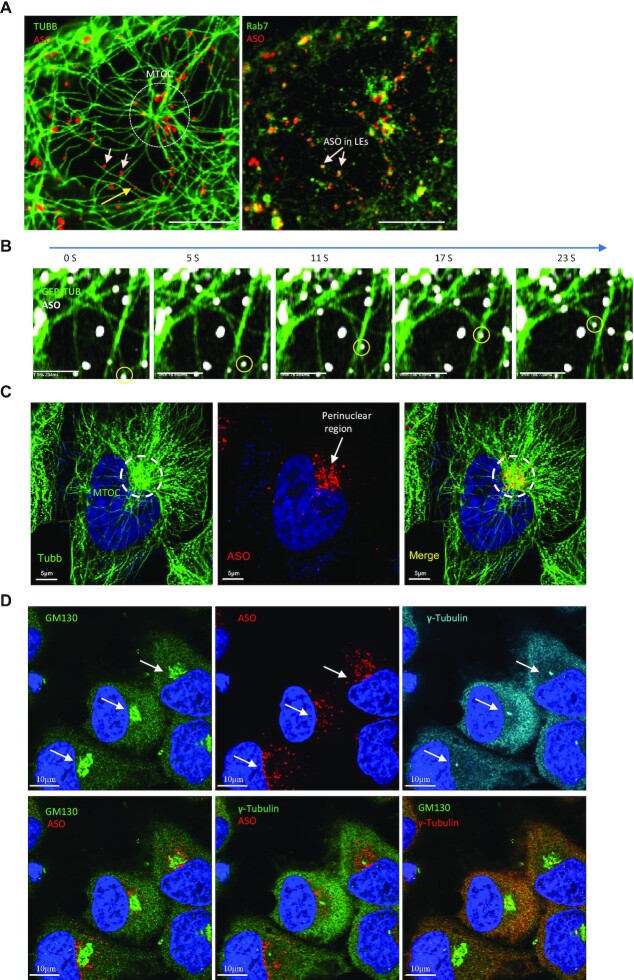
- Liang, X. H., Nichols, J. G., Tejera, D. & Crooke, S. T. Perinuclear positioning of endosomes can affect PS-ASO activities. Nucleic Acids Res 49, 12970-12985 (2021). https://s10-doi-org.libproxy1.nus.edu.sg/nar/gkab1198