Dyne Therapeutics Unveils Positive ACHIEVE and DELIVER Study Results for DYNE-101 in DM1 and DYNE-251 in DMD
Dyne Therapeutics, Inc., a clinical-stage company dedicated to developing groundbreaking treatments for muscle diseases driven by genetic causes, has reported promising clinical results from its ongoing Phase 1/2 ACHIEVE trial involving DYNE-101 in subjects with myotonic dystrophy type 1. Additionally, the company shared positive findings from its continuing Phase 1/2 DELIVER trial of DYNE-251 in patients with Duchenne muscular dystrophy who are suitable for exon 51 skipping.
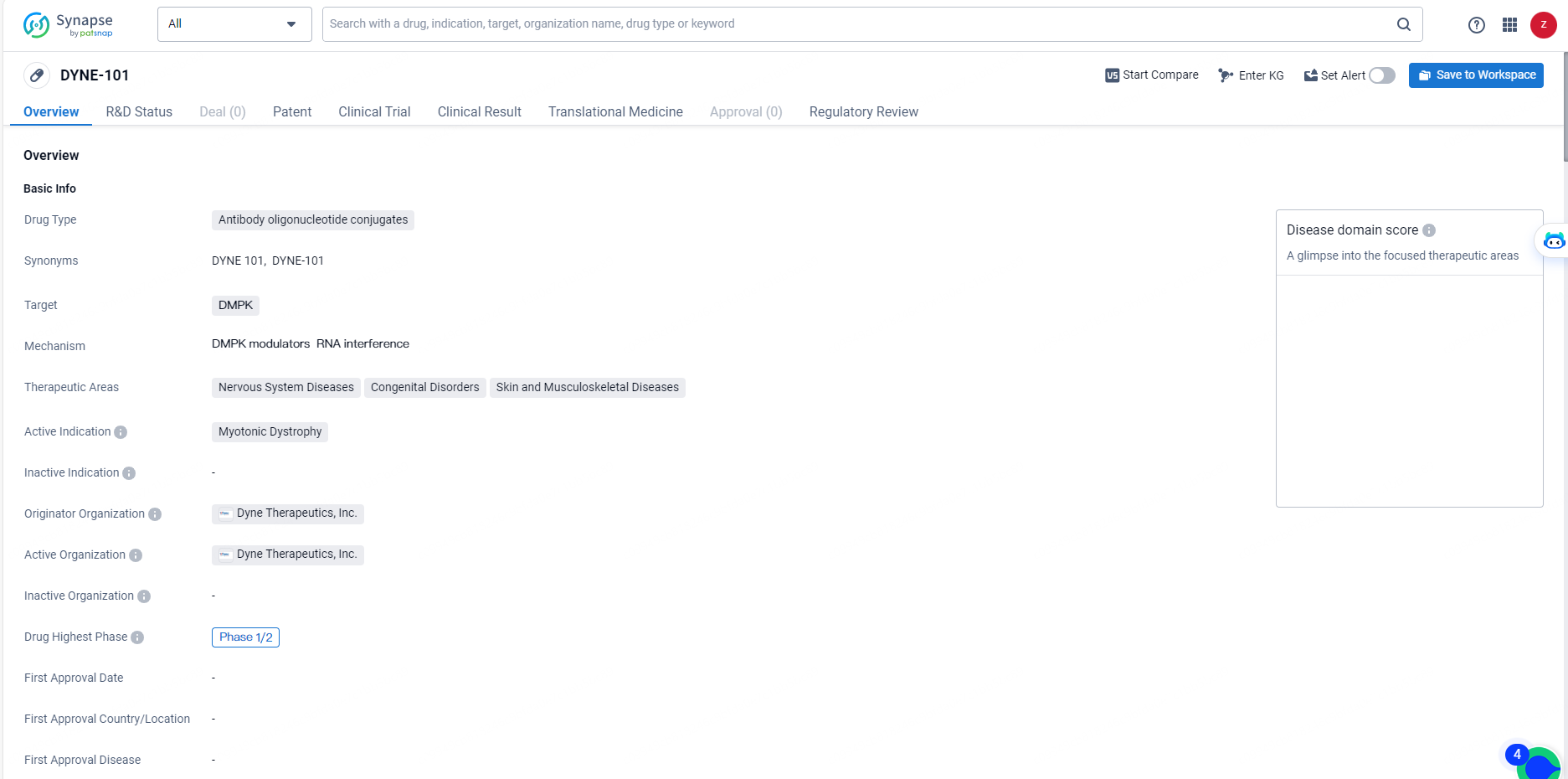
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“We are thrilled to present fresh clinical data from our ACHIEVE and DELIVER studies, revealing significant impacts on key biomarkers and functional enhancements across multiple endpoints that are crucial to patients. We believe these findings showcase the superior potential of our product candidates and underscore the opportunity to revolutionize DM1 and DMD treatments and leverage the FORCE platform for other rare muscle disorders,” stated John Cox, president and CEO of Dyne.
“We think the vast and comprehensive nature of these data is truly distinguishing. Our solid preclinical studies are translating into clinical benefits and indicating favorable safety profiles for both DYNE-101 and DYNE-251,” remarked Wildon Farwell, M.D., MPH, Dyne’s chief medical officer.
“The DM1 and Duchenne communities have long awaited new and improved therapeutic options. Capitalizing on the strength of these promising data and recent regulatory interactions, we are eager to keep engaging with global regulatory bodies this year to pursue accelerated approval pathways and address the urgent unmet medical needs in these communities. We extend our gratitude to the participants, clinicians, and communities involved in these trials for their ongoing partnership,” added Wildon Farwell.
Dyne shared efficacy data from 40 adult DM1 patients in the randomized, placebo-controlled multiple ascending dose segment of the DYNE-101 ACHIEVE study. This included 12-month data for the 1.8 mg/kg Q4W cohort, 6-month data for the 3.4 mg/kg Q4W cohort, and 3-month data for the 5.4 mg/kg Q8W cohort.
In the ACHIEVE study, DYNE-101 exhibited strong muscle delivery and dose-dependent, consistent splicing correction, alongside improvements in several functional endpoints and patient-reported outcomes.
DYNE-101 continued to show dose-dependent splicing correction in subsequent cohorts. Patients in the 5.4 mg/kg Q8W cohort experienced a 27% mean splicing correction from baseline across a comprehensive 22-gene panel over 3 months, with all participants showing splicing correction.
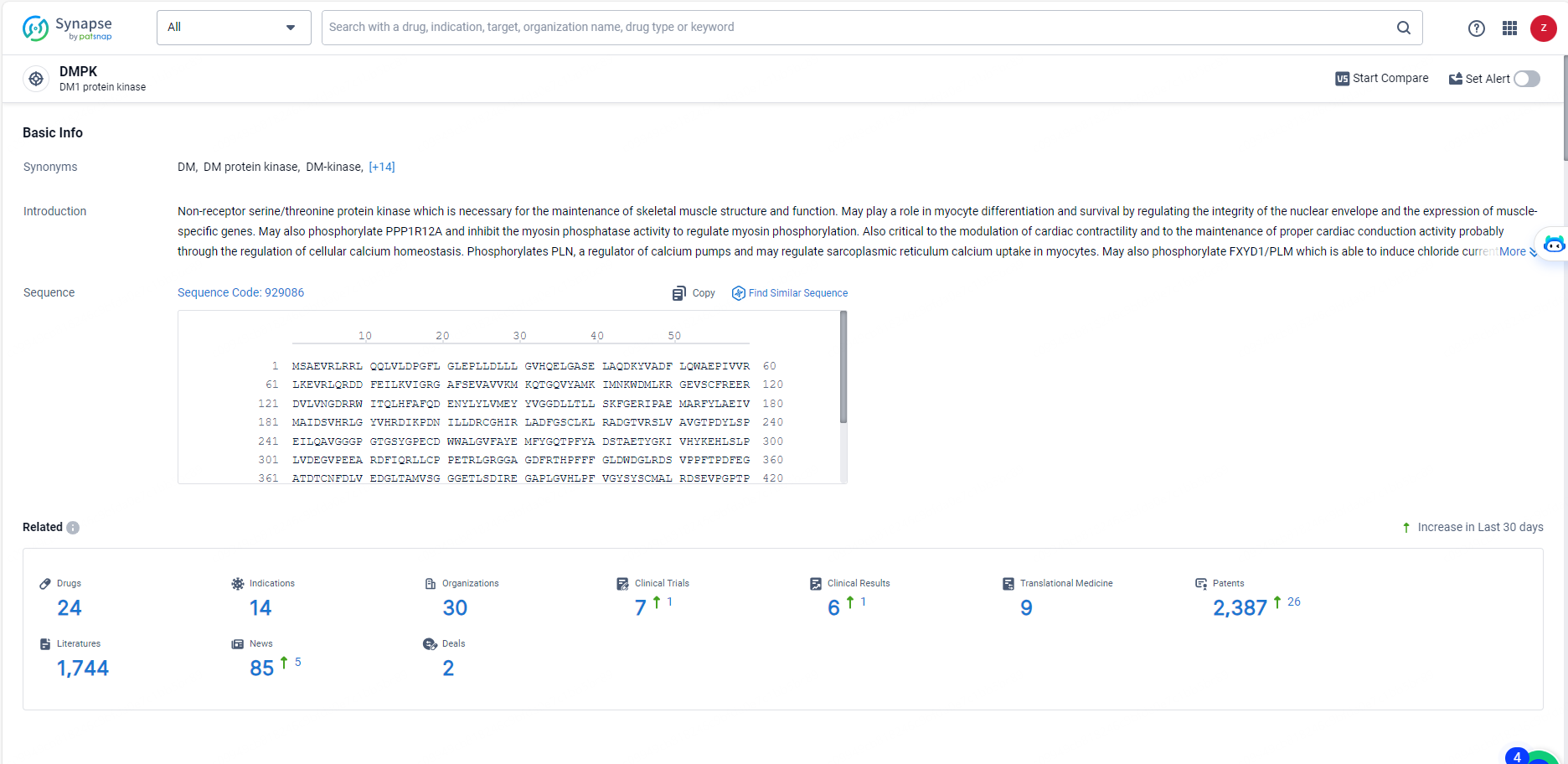
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 27, 2024, there are 24 investigational drugs for the DMPK targets, including 14 indications, 30 R&D institutions involved, with related clinical trials reaching 7, and as many as 2387 patents.
DYNE-101 is an antibody oligonucleotide conjugate drug developed by Dyne Therapeutics, Inc. The drug targets DMPK and is intended for the treatment of Myotonic Dystrophy, a genetic disorder that affects the muscles and other body systems. The therapeutic areas of focus for DYNE-101 include Nervous System Diseases, Congenital Disorders, and Skin and Musculoskeletal Diseases. Its progression to the Phase 1/2 stage and Orphan Drug designation reflect its potential as an innovative and targeted treatment approach in the pharmaceutical industry.