Rapid-Acting Insulin AT278 Proves Superior in Phase I Trial for Overweight Type 2 Diabetics
Arecor Therapeutics plc, a biopharmaceutical company focused on improving modern treatments for better health outcomes, reports that its ultra-concentrated, ultra-rapid acting insulin candidate, AT278, successfully achieved all primary and secondary goals. Additionally, it outperformed NovoRapid and Humulin R U-500 in a Phase I study involving Type 2 diabetic patients with elevated body mass index.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
AT278 is an innovative, highly concentrated insulin formulation that facilitates rapid absorption post-injection, even at high concentrations, thus requiring a smaller injection volume. With no equivalent rapid-acting concentrated insulins currently available, AT278 could be the pioneering solution for patients with substantial daily insulin needs.
Sarah Howell, CEO of Arecor, commented: “We are thrilled with the clinical outcomes from our second Phase I trial, showing AT278's superiority over NovoRapid and Humulin R U-500 in Type 2 diabetes patients with high BMI. These findings corroborate the positive results from our earlier clinical trial in Type 1 diabetes patients, again demonstrating AT278’s superiority. This milestone in AT278’s development reinforces its potential to offer an advanced insulin treatment that reduces patient burden and enhances clinical outcomes for those requiring high daily doses of insulin.”
Many Type 2 diabetes patients with high BMI are inadequately managed with current therapies. Enhanced treatment options like AT278 are needed to alleviate patient burden through fewer daily injections, reduced injection volumes, and flexible dosing around meals. This reduction in patient burden is likely to improve treatment adherence and, combined with AT278’s superior effectiveness, should enhance blood glucose control and overall outcomes. Moreover, a truly ultra-rapid, ultra-concentrated insulin is vital for the advancement of next-generation compact and long-lasting insulin pumps, anticipated to revolutionize diabetes management.
Professor Thomas Pieber, Principal Investigator for the ARE-278-104 clinical trial, stated: “The results are exceptionally significant; AT278 has clearly demonstrated quicker insulin absorption and an accelerated Pharmacokinetic and Pharmacodynamic profile compared to standard lower concentration insulin aspart.”
A comprehensive analysis of the trial data is currently in progress to formulate a strategy for AT278 that maximizes benefits for both shareholders and patients. Detailed trial data will be prepared for publication and presentation at an upcoming international diabetes conference.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
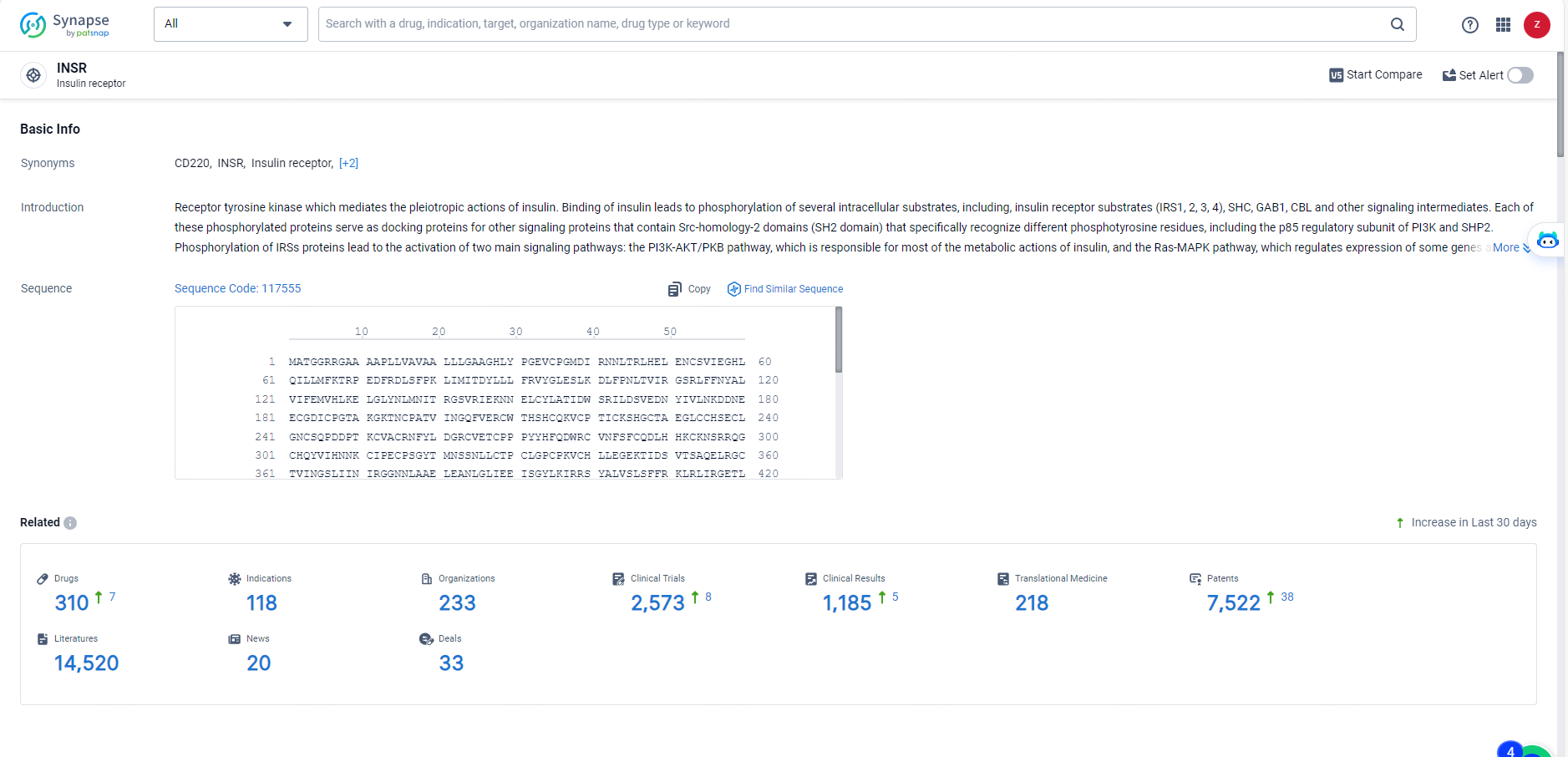
According to the data provided by the Synapse Database, As of May 27, 2024, there are 310 investigational drugs for the INSR target, including 118 indications, 233 R&D institutions involved, with related clinical trials reaching 2573, and as many as 7522 patents.
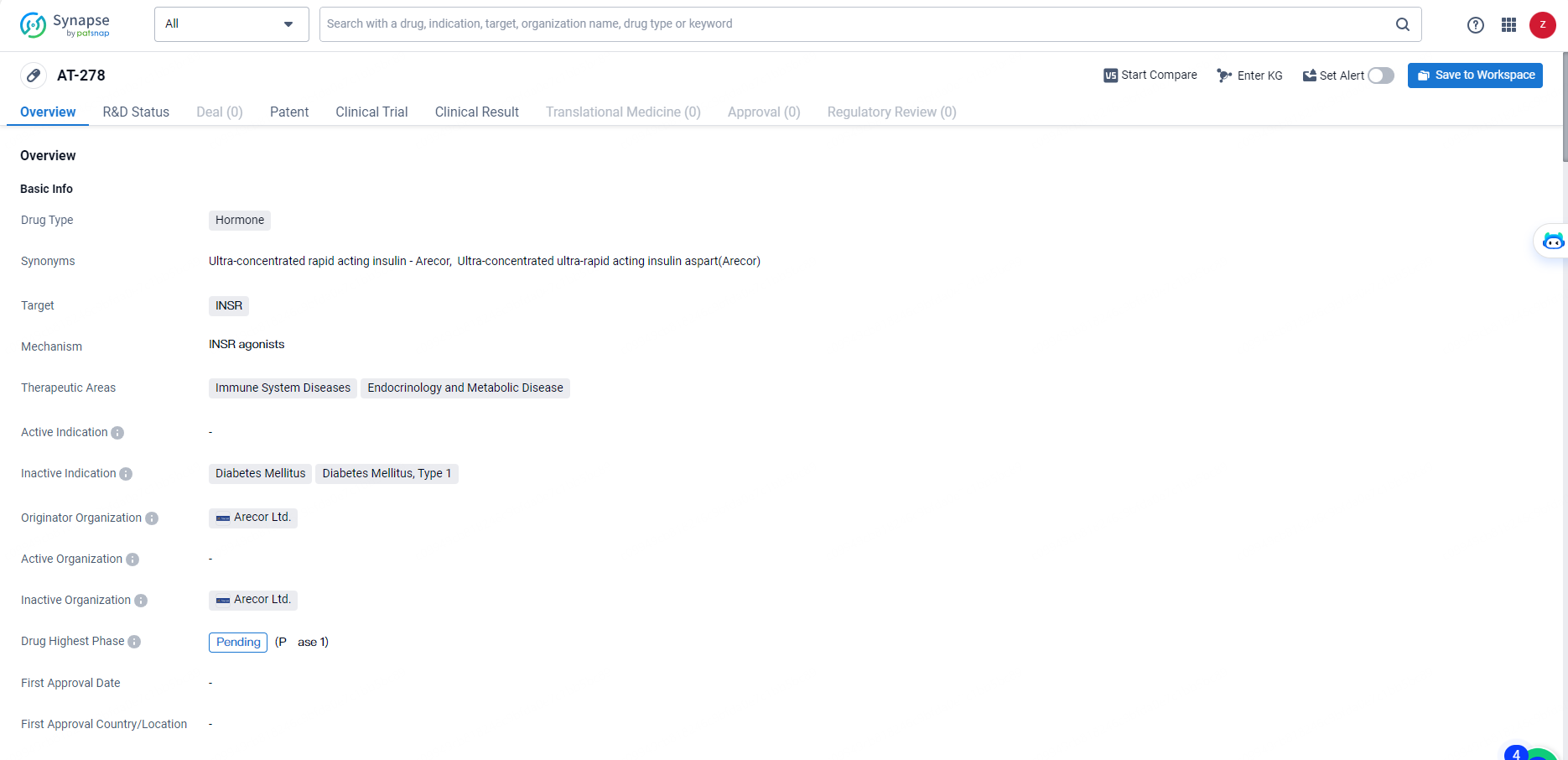
AT-278 is a hormone-based drug developed by Arecor Ltd. The drug targets the INSR, making it relevant for the treatment of immune system diseases and endocrinology and metabolic diseases.
As the highest phase for AT-278 is pending, it will be important to monitor the progress of this drug in the pharmaceutical industry and its potential impact on the treatment of these medical conditions.