GRI Bio's GRI-0621 Shows Promise in Preclinical IPF Study
GRI Bio, Inc., a biotech firm developing a groundbreaking range of Natural Killer T cell modulators aimed at addressing inflammatory, fibrotic, and autoimmune disorders, has today unveiled promising preclinical results. These findings highlight that its leading candidate, GRI-0621, effectively diminishes key inflammatory and fibrotic factors in Idiopathic Pulmonary Fibrosis.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
During the 2024 ATS International Conference, which took place from May 17-22, 2024, in San Diego, CA, Dr. Albert Agro, Chief Medical Officer at GRI Bio, showcased a poster titled “Altered NKT Cell Populations in the Airways of Patients With IPF.” The poster included translational data from IPF patients and highlighted findings from a bleomycin-induced fibrosis model in mice, indicating that GRI-0621, a selective iNKT cell inhibitor, can modulate fibrotic conditions and diminish key inflammatory and fibrotic drivers of the disease. The poster also detailed the design of a Phase 2a study to evaluate the safety, tolerability, and biomarker effects of GRI-0621.
Dr. Agro remarked, “This data enhances our confidence in GRI-0621’s potential as a treatment for IPF. Our commitment to the potential of our NKT platform technology is growing as we aim to develop novel biomarkers and therapeutic targets for differentiating stages of fibrosis progression. We remain dedicated to advancing our Phase 2a biomarker study of GRI-0621 and eagerly anticipate the interim and topline data in the upcoming quarters to further explore its potential benefits for patients.”
GRI-0621 is characterized as a small molecule RAR-βɣ inhibitor targeting iNKT cell activity by binding to RAR receptors, which are expressed at higher levels in iNKT cells compared to other T cell populations examined.
In an IPF animal model, GRI-0621 was effective in reducing the fibrotic score, inhibiting TGFβ and VCAM-1 production, and decreasing immune cell infiltration.
IPF is a rare, chronic, and progressively worsening lung disease marked by abnormal scarring that impedes oxygen transfer to the bloodstream. Currently, the treatment options for IPF are restricted to two approved drugs, which are associated with significant side effects, limited patient compliance, and no survival benefit.
GRI-0621, GRI Bio’s leading candidate, is a small molecule RAR-βɣ dual agonist that inhibits the activity of human iNKT cells. Previous and early clinical trials with the oral formulation of GRI-0621 have demonstrated its ability to ameliorate fibrosis in various disease models, and improve liver function tests and other inflammation and injury markers in patients.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
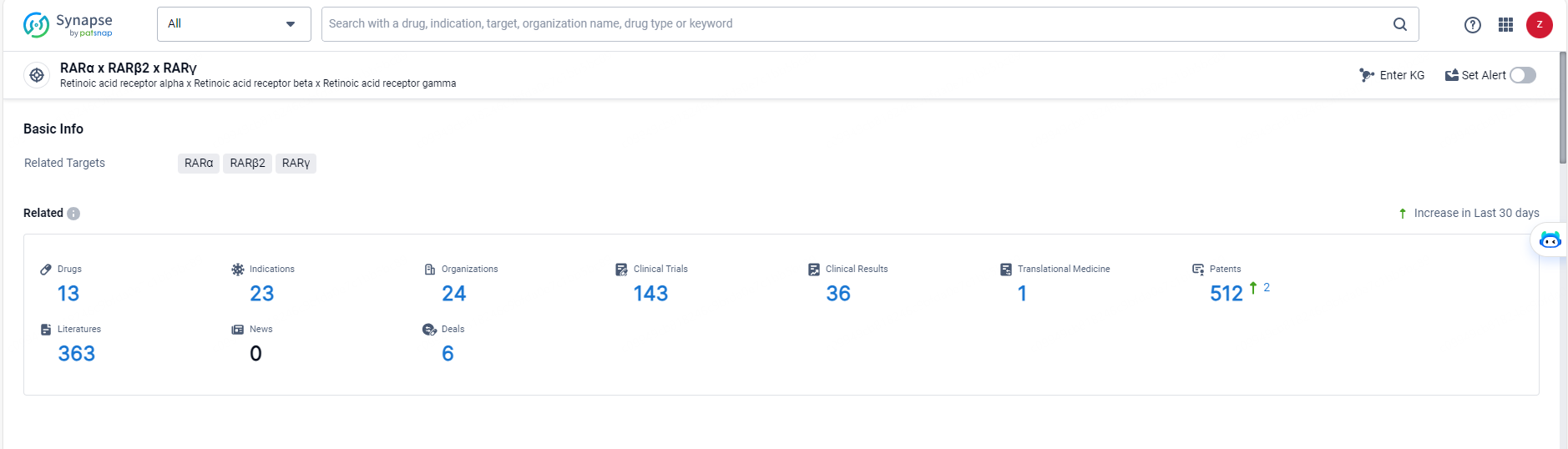
According to the data provided by the Synapse Database, As of May 27, 2024, there are 13 investigational drugs for the RAR-βɣ target, including 23 indications, 24 R&D institutions involved, with related clinical trials reaching 143, and as many as 512 patents.
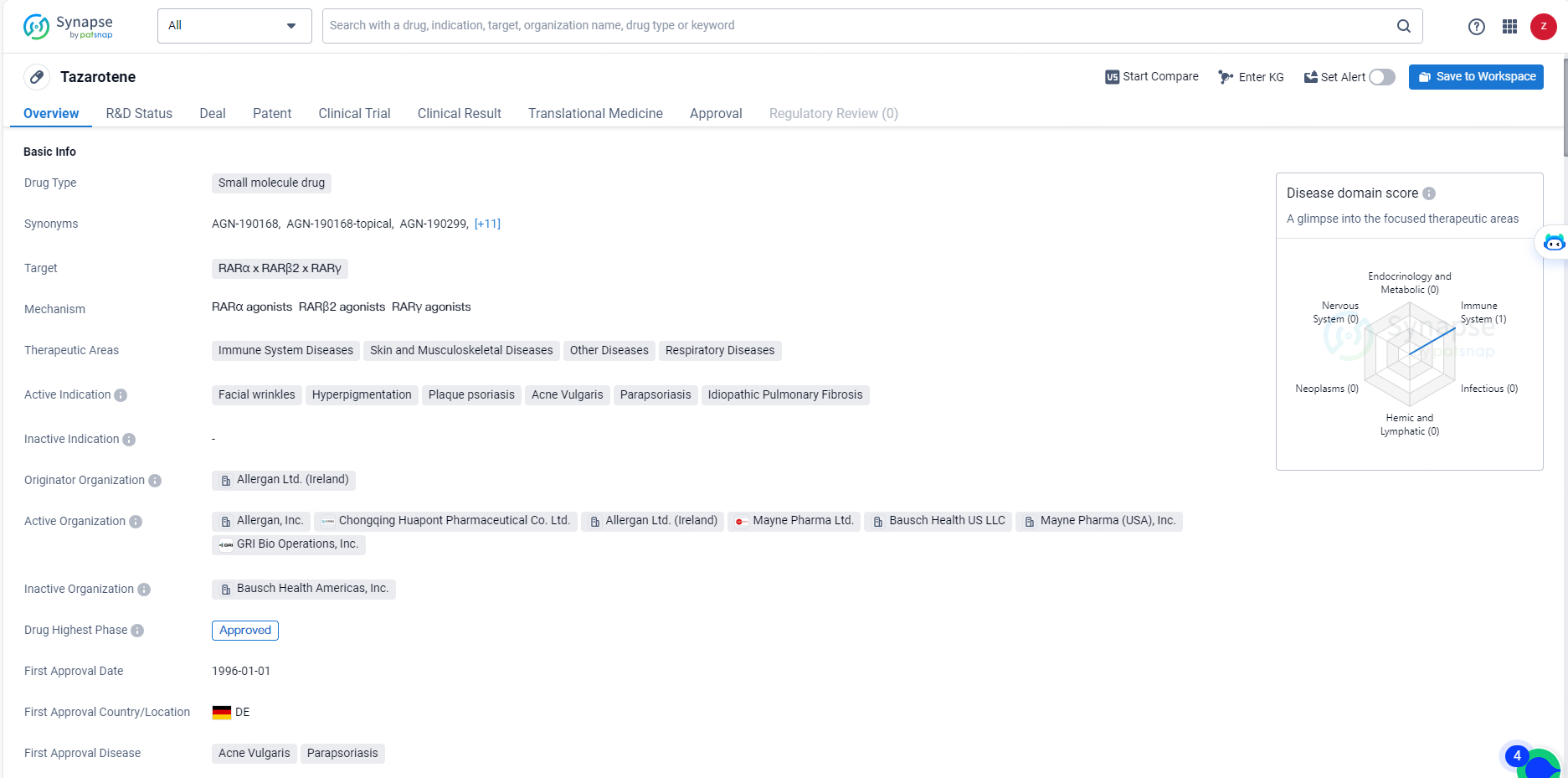
Tazarotene's approval and therapeutic indications position it as a valuable asset in the pharmaceutical industry, with the potential to address a wide range of medical conditions. Its approval in multiple markets and its origin from a reputable organization further solidify its status as a significant drug in the field of biomedical research and therapeutic development.