Is Monomethyl fumarate approved by the FDA?
Yes, Monomethyl fumarate, known by the brand name Bafiertam, is FDA approved. The FDA approved Bafiertam for the treatment of relapsing forms of multiple sclerosis (MS) on April 28, 2020.
What is Monomethyl Fumarate?
Monomethyl fumarate is a medication used to treat relapsing forms of multiple sclerosis (MS) in adults. These forms include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease. It is classified as a selective immunosuppressant.
How Does Monomethyl Fumarate Work?
Monomethyl fumarate works by modulating the immune response, which is believed to help reduce the frequency of relapse and slow the progression of disability in people with multiple sclerosis. It is administered in the form of oral delayed-release capsules.
Dosage and Administration
Monomethyl fumarate is taken orally, with or without food. The typical dosing regimen involves a "starter dose" of 95 mg taken twice daily for the first 7 days, followed by a "maintenance dose" of 190 mg taken twice daily thereafter. It is important to swallow the capsules whole without crushing, chewing, or opening them.
Side Effects
Common side effects of monomethyl fumarate include:
- Flushing (sudden warmth, redness, or tingling sensation)
- Stomach pain, indigestion
- Nausea, vomiting
- Diarrhea
Serious side effects can occur, and it is important to contact a healthcare provider if you experience:
- Signs of an allergic reaction (hives, difficulty breathing, swelling of face, lips, tongue, or throat)
- Severe warmth, redness, burning, or itching under the skin
- Liver problems (loss of appetite, stomach pain, tiredness, dark urine, jaundice)
- Signs of infection (fever, chills, sweating, mouth sores, headache, confusion, neck stiffness, sensitivity to light, vomiting, severe diarrhea)
- Symptoms of a herpes virus infection (flu-like symptoms, cold sores around the mouth, blistering rash, burning pain in the thigh or lower back)
Precautions
Before taking monomethyl fumarate, inform your doctor if you have had:
- Low white blood cell counts
- Any infection
- Liver disease
Monomethyl fumarate is not recommended for individuals younger than 18 years old, or for those concurrently using dimethyl fumarate or diroximel fumarate.
Storage
Store unopened bottles of monomethyl fumarate capsules in a refrigerator, but do not freeze them. Once opened, store the bottle at room temperature away from moisture, heat, and light, and keep the capsules in the original bottle. Unused capsules should be discarded 3 months after first opening the bottle.
Conclusion
Monomethyl fumarate, approved by the FDA on April 28, 2020, is a significant medication for managing relapsing forms of multiple sclerosis. Its ability to modulate the immune system helps reduce relapse rates and manage the progression of the disease. As with any medication, it is crucial to follow the prescribed dosing regimen and consult with a healthcare provider about any side effects or concerns.
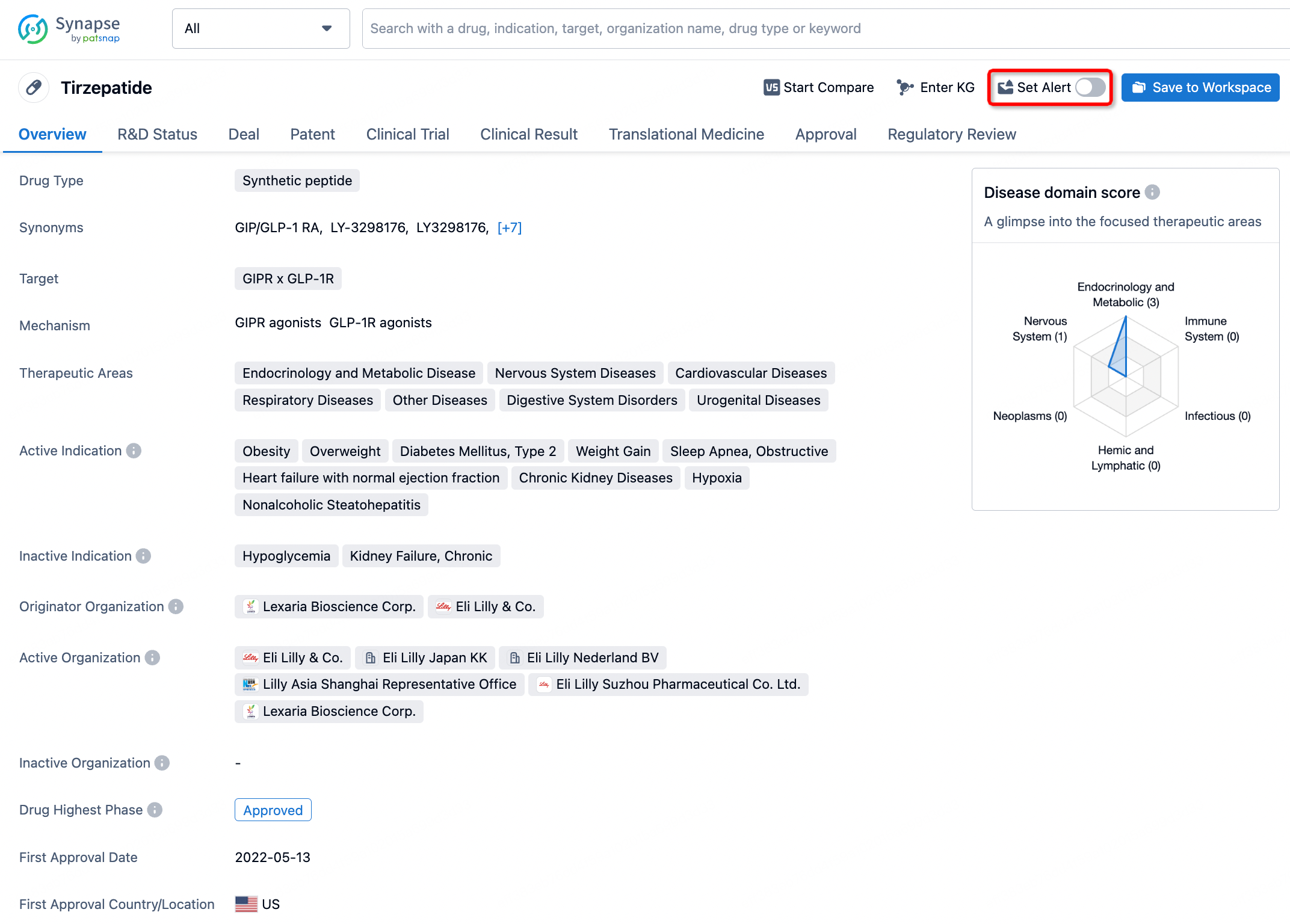
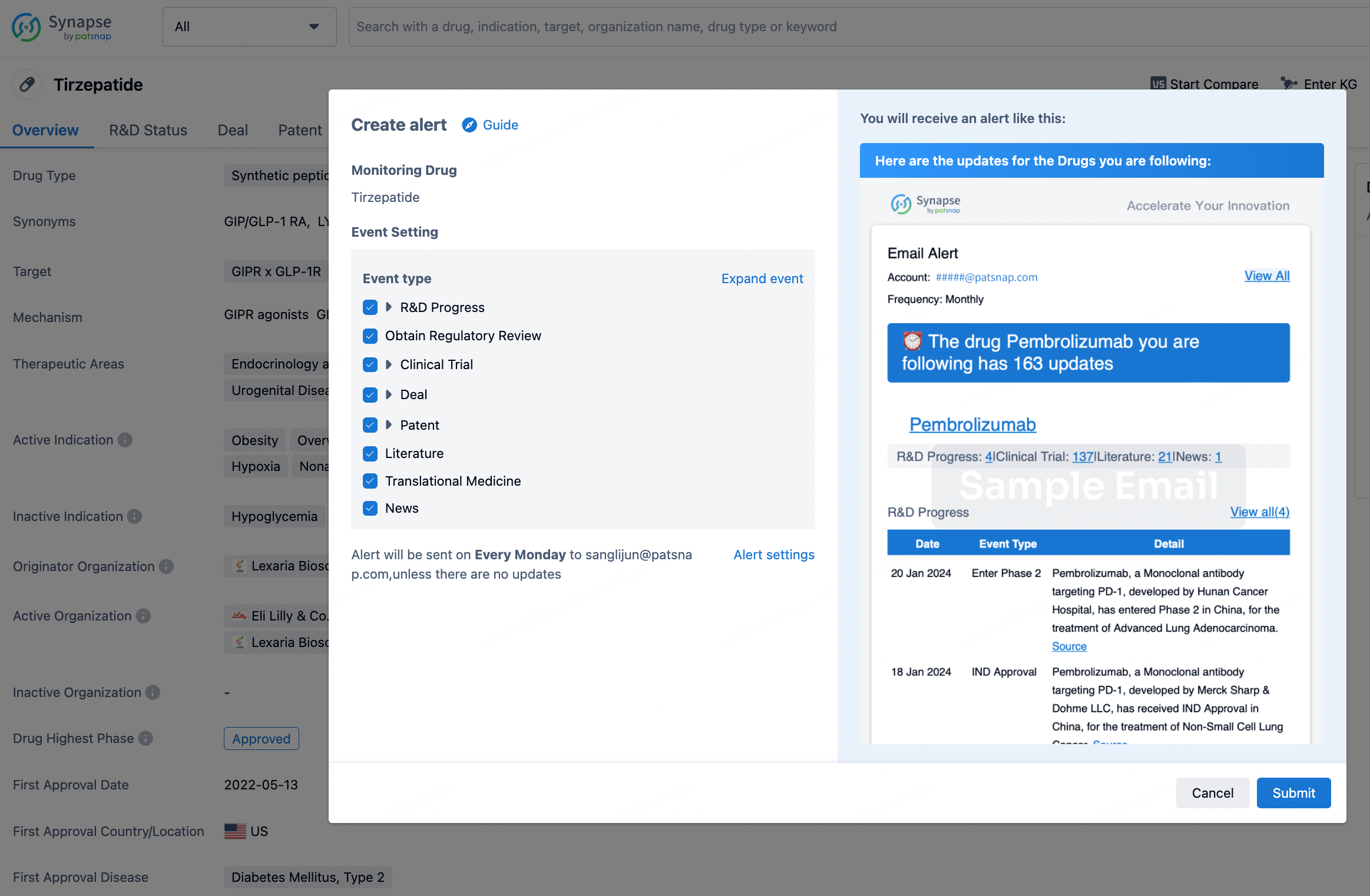
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!