vTv Therapeutics Begins Key CATT1 Study Enrollment for Cadisegliatin in Type 1 Diabetes
vTv Therapeutics Inc., a biopharmaceutical firm in advanced stages and with a novel clinical portfolio of small molecules, primarily focused on diabetes, has revealed that the initial patient has undergone screening in their CATT1 key trial. This study is assessing the use of cadisegliatin as a complementary therapy for type 1 diabetes.
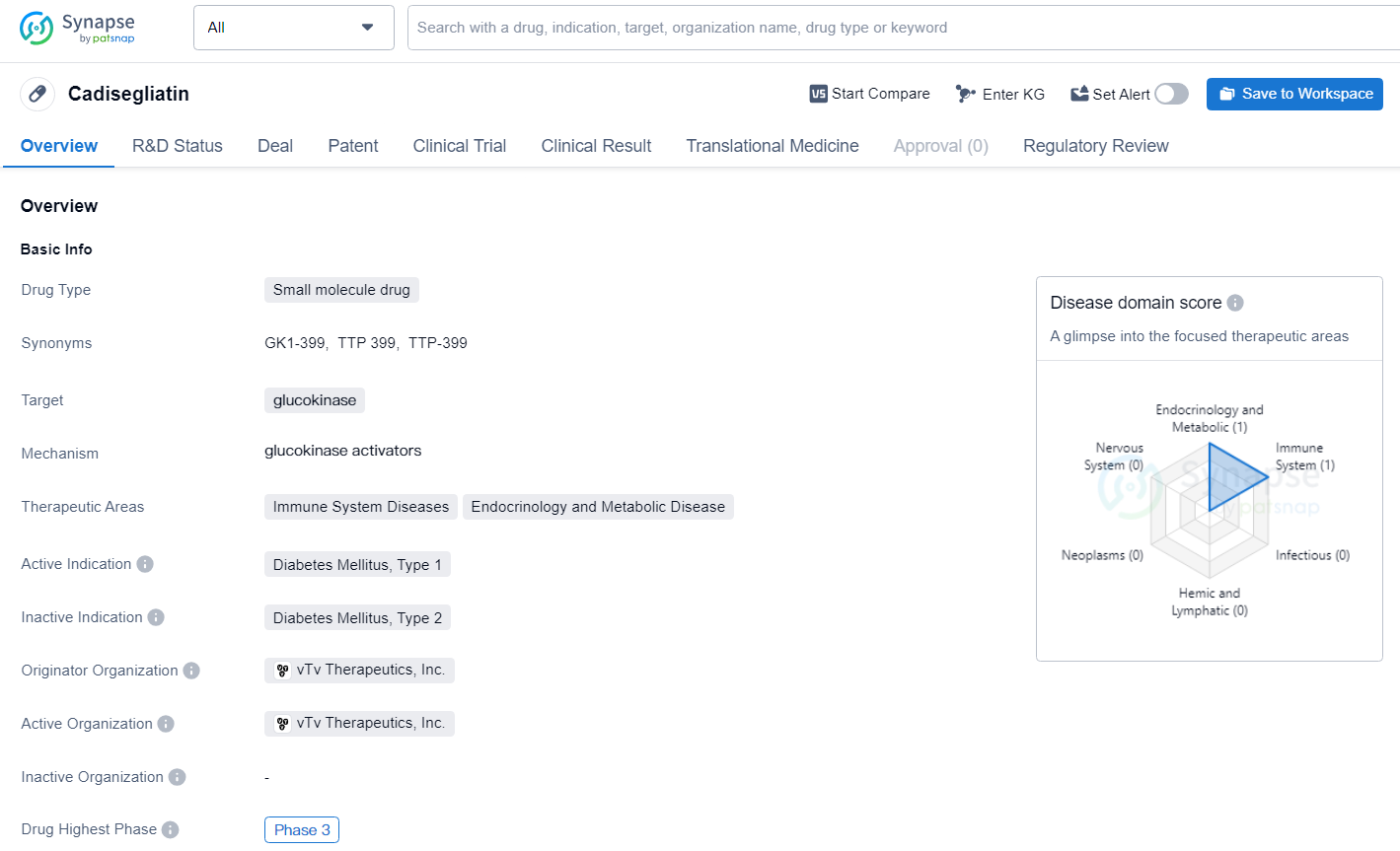
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
CATT1 is structured as a registrational study, forming a central component of the regulatory filing for cadisegliatin. This investigational drug is a potentially groundbreaking oral, liver-specific glucokinase activator for T1D. So far, it has been administered to over 500 participants, including 300 individuals with T1D and type 2 diabetes.
"Achieving glycemic control is a significant challenge for nearly 8 million people with T1D globally. Cadisegliatin represents a novel strategy designed to manage blood glucose levels by targeting glucose pathways specifically in the liver," stated Paul Sekhri, Chairman, President, and CEO of vTv Therapeutics. "Enrolling the first patient is a crucial step forward for our advanced-stage cadisegliatin program, bringing us closer to our goal of offering innovative therapies that enhance the quality of life for millions living with diabetes."
Dr. Thomas Strack, Chief Medical Officer at vTv Therapeutics, commented, "85% of T1D patients experience one or two hypoglycemic events per week, which is a significant hurdle to optimal treatment. Cadisegliatin is devised to restore the liver’s glucose management independently of insulin, aiming to safely manage hyper- and hypoglycemic episodes in diabetic patients."
In addition, cadisegliatin will be investigated as an add-on therapy to insulin in T2D patients in an upcoming Phase 2 trial scheduled for the Middle East, in partnership with G42 Healthcare Research Technology Projects LLC and IROS, a clinical research organization based in the UAE. This trial is projected to commence in the latter half of 2024.
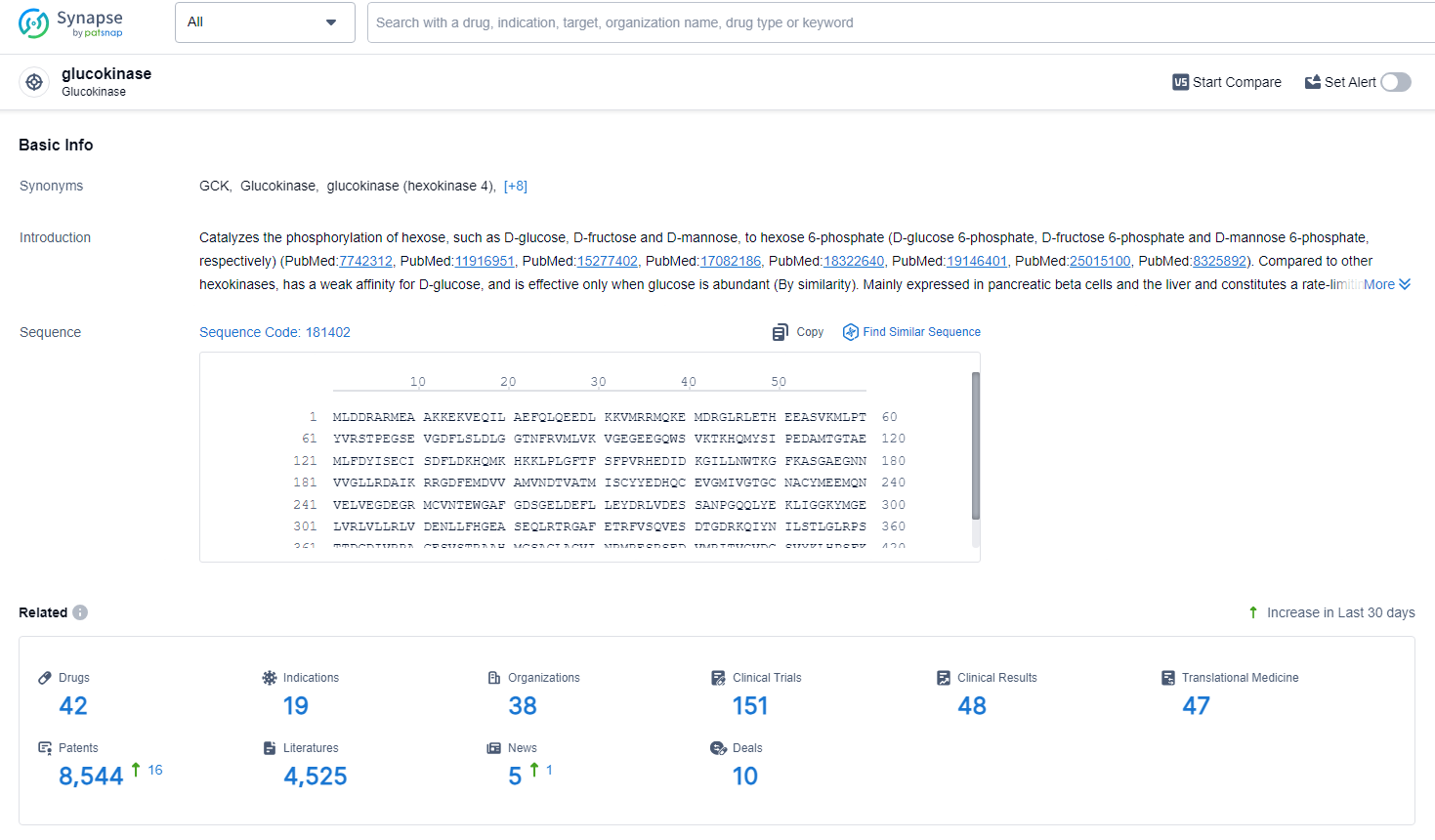
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 1, 2024, there are 42 investigational drugs for the glucokinase target, including 19 indications, 38 R&D institutions involved, with related clinical trials reaching 151, and as many as 8544 patents.
Cadisegliatin targets glucokinase for the treatment of Diabetes Mellitus, Type 1. With its Breakthrough Therapy designation and advancement to Phase 3 of development, Cadisegliatin holds promise as a potential therapeutic option for individuals with Type 1 Diabetes, and its progress reflects the ongoing efforts in the pharmaceutical industry.