Electra Therapeutics Reports Positive Phase 1b Results for ELA026 in sHLH at ASH 2024
Electra Therapeutics, Inc., a clinical-stage biotech firm specializing in antibody therapies for novel targets in immunological disorders and cancer, has reported favorable outcomes from the concluded Phase 1b trial of ELA026 for treating secondary hemophagocytic lymphohistiocytosis (sHLH). ELA026 is a pioneering monoclonal antibody that binds to SIRP-α/β1/γ on the surface of myeloid cells and T lymphocytes, which are the primary immune cells causing cytokine storms and hyperinflammation in sHLH. By targeting SIRP to selectively eliminate pathogenic immune cells, ELA026 holds promise for wide-ranging applications in immunology, inflammation, and cancer. The ELA026 study findings for sHLH will be presented today at 2:45 PM PST during an oral session at the American Society of Hematology (ASH) Annual Meeting in San Diego, CA.
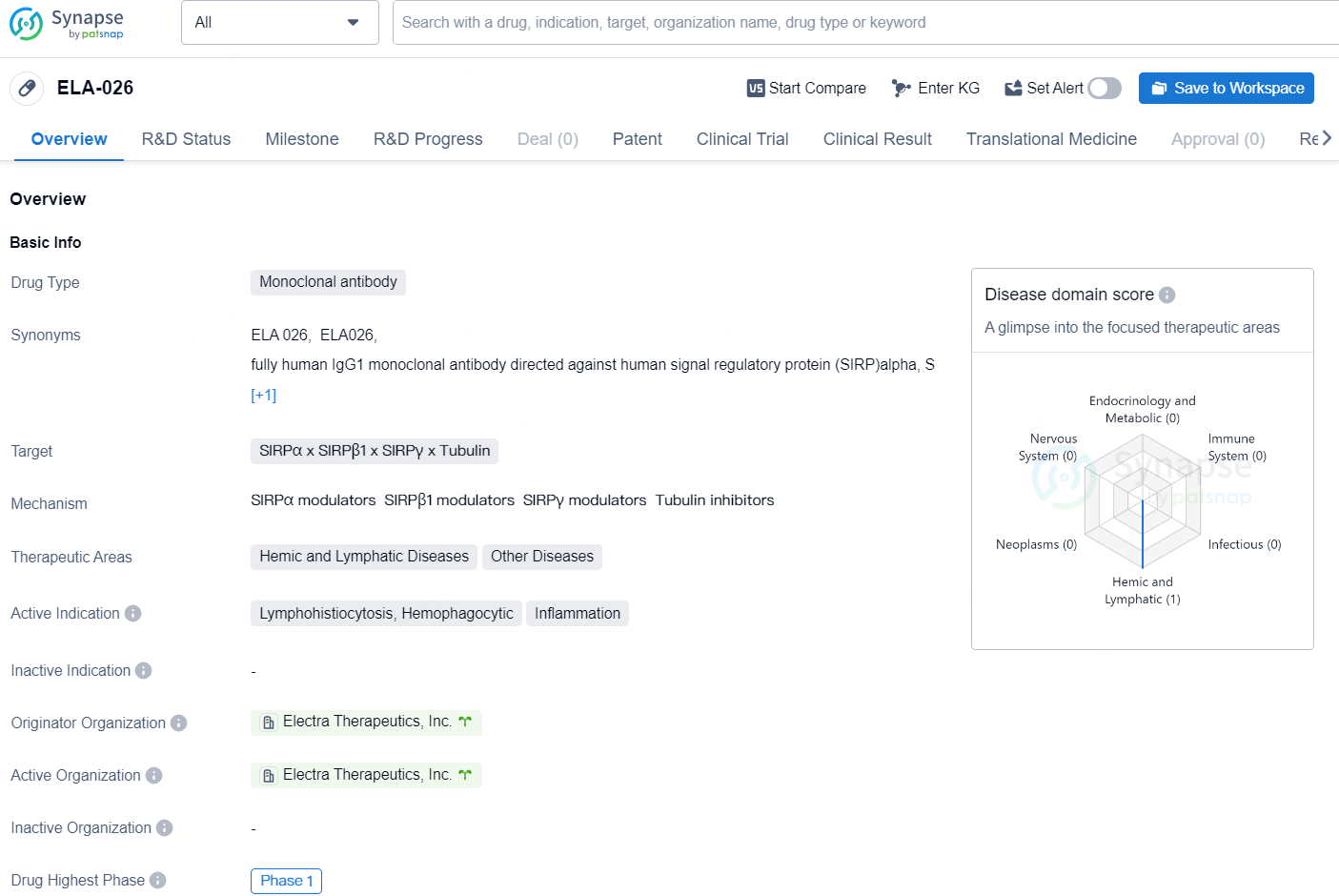
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Phase 1b clinical trial was an open-label, single-arm, multicenter study aimed at assessing the safety and efficacy of ELA026, examining its pharmacodynamic (PD) and pharmacokinetic (PK) profiles, and determining a dosing regimen for future Phase 2/3 studies (NCT05416307). The study results indicated that for sHLH patients with the worst prognosis, specifically those with malignancy-associated HLH (mHLH), ELA026 treatment in frontline settings achieved a 100% overall response rate by week 4, 100% hospital discharge, and 92% survival at two months. Various PD and HLH-related biomarkers demonstrated that ELA026 rapidly reduced inflammation, aligning with clinical responses.
"ELA026 has demonstrated the potential to be a game-changing treatment for sHLH, a life-threatening condition without approved therapies. Compared to current treatments, which may involve chemotherapy and single cytokine-directed therapies, ELA026 could provide a safer and more effective method to manage the intense immune response in sHLH. The Phase 1b study results, showing rapid PD and biomarker effects along with improved survival at two months, are particularly encouraging," stated Swaminathan P. Iyer, MD, Professor in the Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center. "There is a growing consensus that controlling the cytokine storm early in the sHLH disease course is essential to prevent multiorgan failure and allow for appropriate treatment of the underlying cancer. It is evident that managing this critical early period of hyperinflammation in sHLH can provide significant clinical benefits for patients."
"We are extremely encouraged by these impressive results for sHLH patients, and Electra is committed to advancing ELA026 into a pivotal study as quickly as possible. With determination and focus, our team has developed a drug program targeting SIRP, moving from a novel concept to pivotal study readiness in just five years," said Kathy Dong, PharmD, MBA, President and CEO of Electra Therapeutics. "We have shown the therapeutic value of selectively depleting pathological immune cells to modulate inflammation and are excited to apply this innovative approach with ELA026 and our pipeline to address other diseases with high unmet needs."
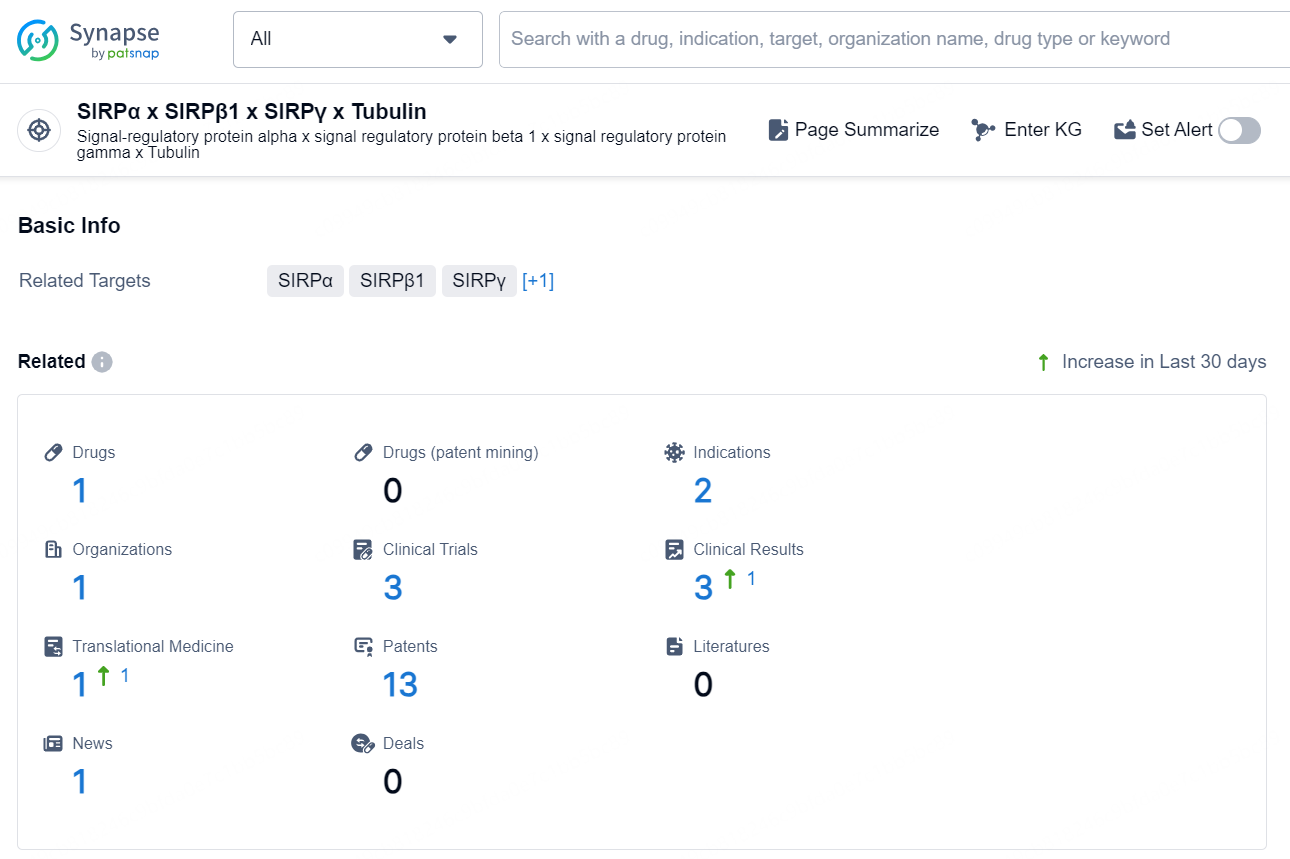
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 13, 2024, there are 1 investigational drug for the SIRPα x SIRPβ1 x SIRPγ x Tubulin target, including 2 indications, 1 R&D institution involved, with related clinical trials reaching 3, and as many as 13 patents.
ELA-026 is a monoclonal antibody drug developed by Electra Therapeutics, Inc. The drug targets SIRPα, SIRPβ1, SIRPγ, and Tubulin, and is intended to treat hemic and lymphatic diseases as well as other diseases. The active indications for ELA-026 are lymphohistiocytosis, hemophagocytic, and inflammation.