Eli Lilly: GLP-1R/GCGR/GIPR Triagonist Reduces Liver Fat Content by 86%
On June 10th, researchers from Eli Lilly published findings of a randomized phase 2a clinical trial in "Nature Medicine" titled “Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial.” The study indicated that Retatrutide (LY3437943), a GLP-1R-related triple receptor agonist currently under clinical development, demonstrated significant efficacy and favorable safety in the treatment of metabolic dysfunction-associated steatotic liver disease (MASLD).

Metabolic dysfunction-associated steatotic liver disease (MASLD), also known as nonalcoholic fatty liver disease (NAFLD), has seen its global prevalence surge from 25% between 1990 and 2006 to 38% between 2016 and 2019. Obesity is one of the main contributors to the increased burden of MASLD, with at least half of MASLD patients also suffering from obesity. The progressive form of MASLD, known as nonalcoholic steatohepatitis (NASH), is characterized by liver inflammation, hepatocellular injury, and fibrosis, and may eventually progress to cirrhosis.
According to previous reports by GPCR3D, Retatrutide (LY3437943) is a triple agonist peptide developed by Eli Lilly that targets the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR), and glucagon-like peptide-1 receptor (GLP-1R). Retatrutide activates human GCGR, GIPR, and GLP-1R with EC50 values of 5.79, 0.0643, and 0.775 nM, respectively.

Studies indicate that GLP-1R (glucagon-like peptide-1 receptor), GIPR (glucose-dependent insulinotropic polypeptide receptor), and GCGR (glucagon receptor) act as crucial "regulators" in maintaining human blood glucose balance and are important therapeutic targets for type 2 diabetes and obesity. Specifically, GLP-1R promotes insulin secretion, lowers blood glucose, and reduces body weight; GIPR increases insulin secretion during hyperglycemia and stimulates glucagon release during hypoglycemia; while GCGR is involved in the regulation of blood glucose and energy. Retatrutide (LY3437943) could be utilized for research on obesity, diabetes, and related conditions (such as non-alcoholic steatohepatitis, NASH).
In September 2022, Eli Lilly published the results of its preclinical studies on LY3437943 in the journal Cell Metabolism, in an article titled "LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept."

In vitro and in vivo validation of the triple agonist effect of LY3437943:

Eli Lilly's research results show that LY3437943 demonstrates superior weight loss effects compared to GLP-1 receptor (GLP-1R) selective agonists (GLP-1 RA), GLP-1R and GCGR dual agonists (Cotadutide or 05677), and GLP-1R and GIPR dual agonists.
LY3437943 reduces fasting glucose and insulin levels in obese mice, indicating an improvement in insulin sensitivity.

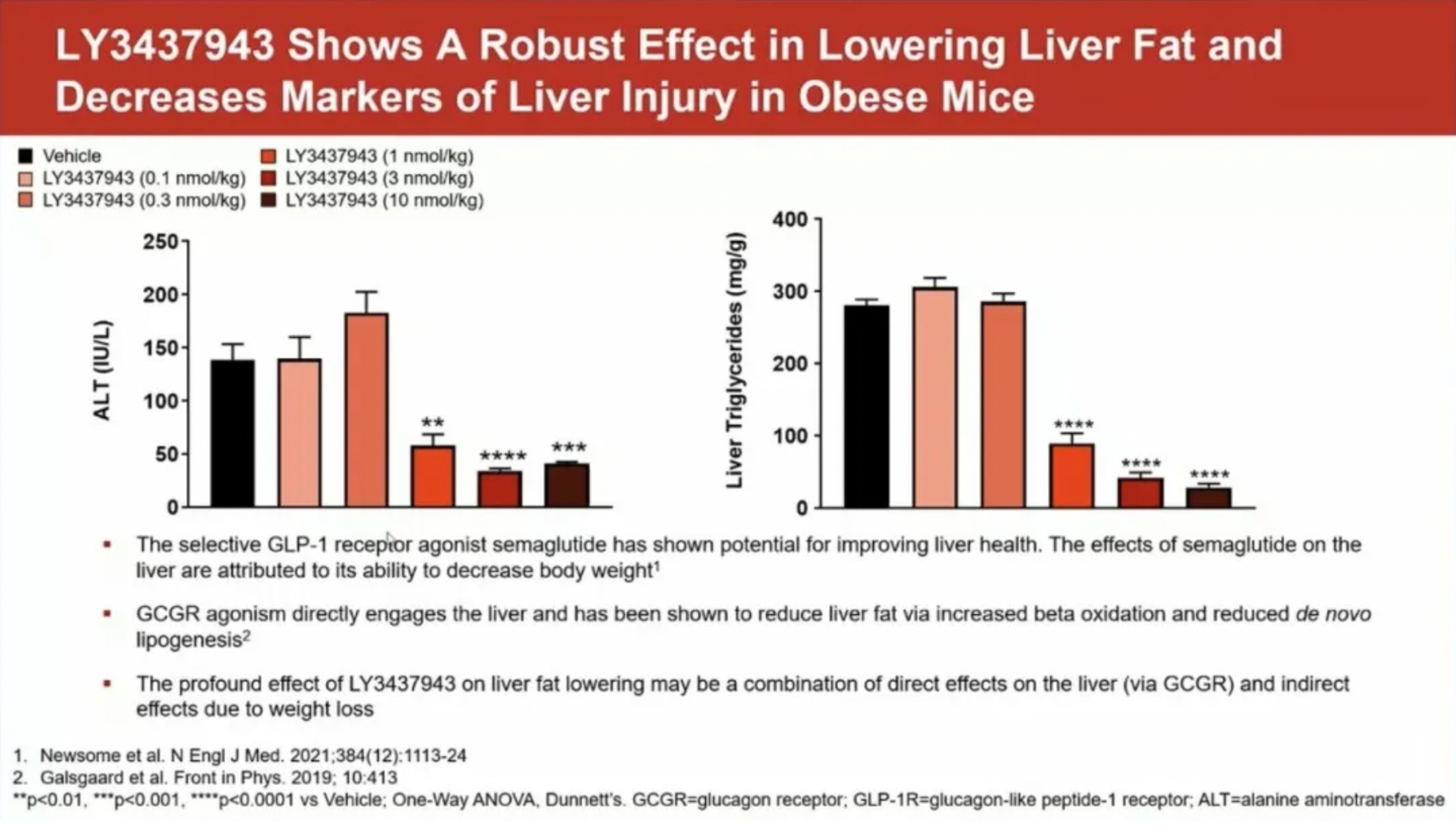
LY3437943 has shown a powerful effect in reducing liver fat and liver injury markers in obese mice, which may be attributed to a combination of GCGR's direct impact on the liver or the indirect effects resulting from weight loss.
In June 2023, The New England Journal of Medicine published Eli Lilly and Company's Phase 2 clinical trial data on Retatrutide (LY3437943) online. The publication is titled "Triple-Hormone-Receptor Agonist Retatrutide for Obesity––––A Phase 2 Trial."
In this study, 338 obese patients received weekly subcutaneous injections of Retatrutide or a placebo. The study evaluated body weight changes over 24 and 48 weeks across various treatment groups, along with a series of metabolic indicators such as glycated hemoglobin and triglyceride levels.
Regarding safety, the most common adverse events associated with Retatrutide were gastrointestinal-related. These events were dose-dependent, primarily of mild to moderate severity, and were partially alleviated by lower initial doses (2 mg vs. 4 mg).
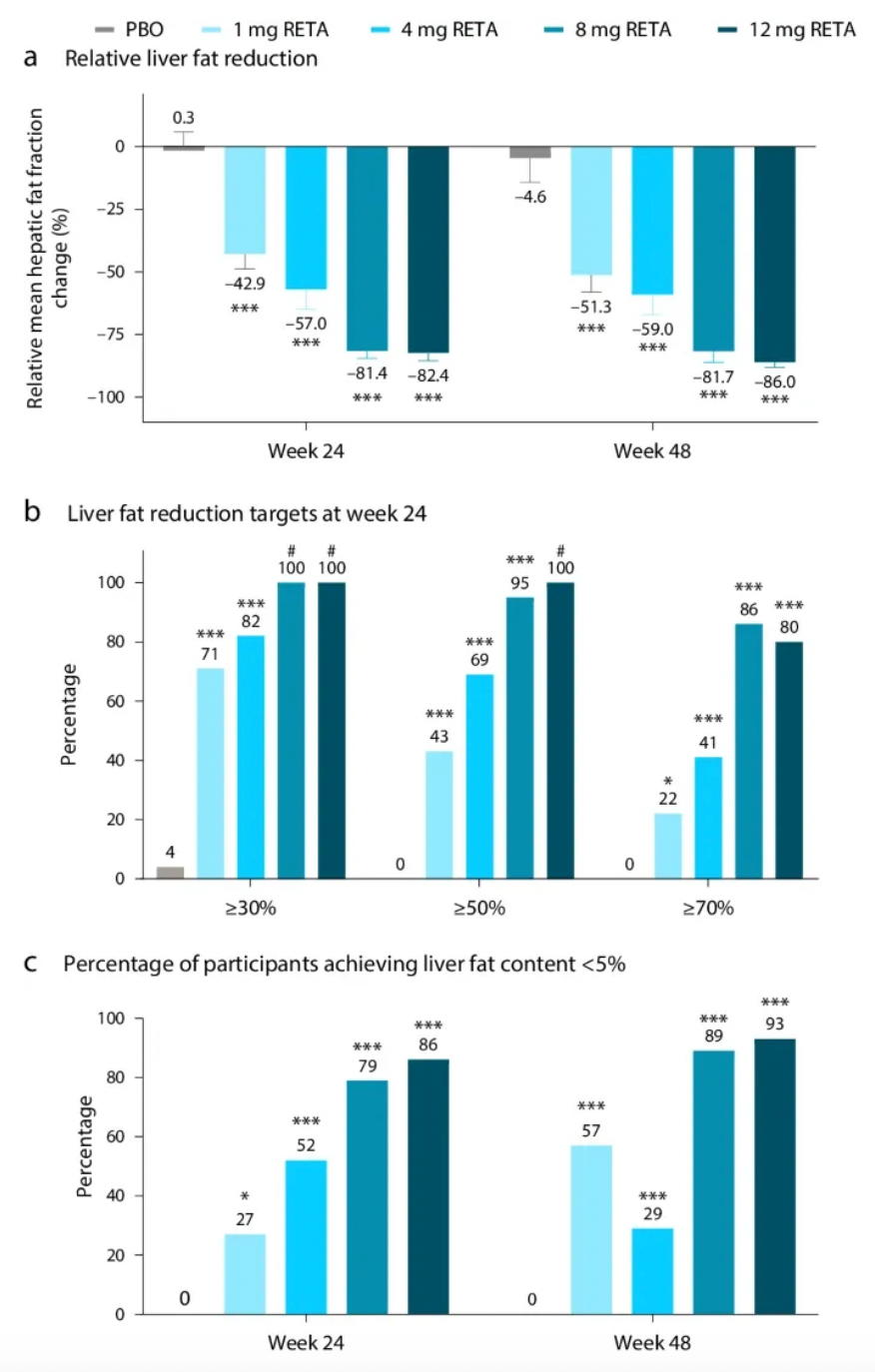
In terms of efficacy, after 24 weeks of treatment, the changes in body weight for different doses in the Retatrutide group were: -2.1% for the placebo group, -7.2% for the 1 mg group, -12.9% for the 4 mg group, -17.3% for the 8 mg group, and -17.5% for the 12 mg group. After 48 weeks of treatment, the results were: -2.1% for the placebo group, -8.7% for the 1 mg group, -17.1% for the combined 4 mg group, -22.8% for the combined 8 mg group, and -24.2% for the 12 mg group.
Lilly's recently released research data indicates that all doses of Retatrutide significantly reduced liver fat content at week 48 compared to the placebo group. Notably, in the 8 mg and 12 mg dose groups, over 85% of participants achieved a significant reduction in liver fat content, defined as a total liver fat content of less than 5%. Furthermore, Retatrutide treatment was closely associated with reductions in body weight, waist circumference, visceral adipose tissue (VAT), and abdominal subcutaneous adipose tissue (ASAT), as well as improvements in insulin sensitivity and lipid metabolism markers.
In terms of safety, the safety profile of Retatrutide in patients with MASLD is similar to that observed in the broader population with obesity. Most adverse events were mild to moderate gastrointestinal events, primarily occurring during the dose-escalation period. No drug-induced serious hepatotoxicity signals were observed in the study.
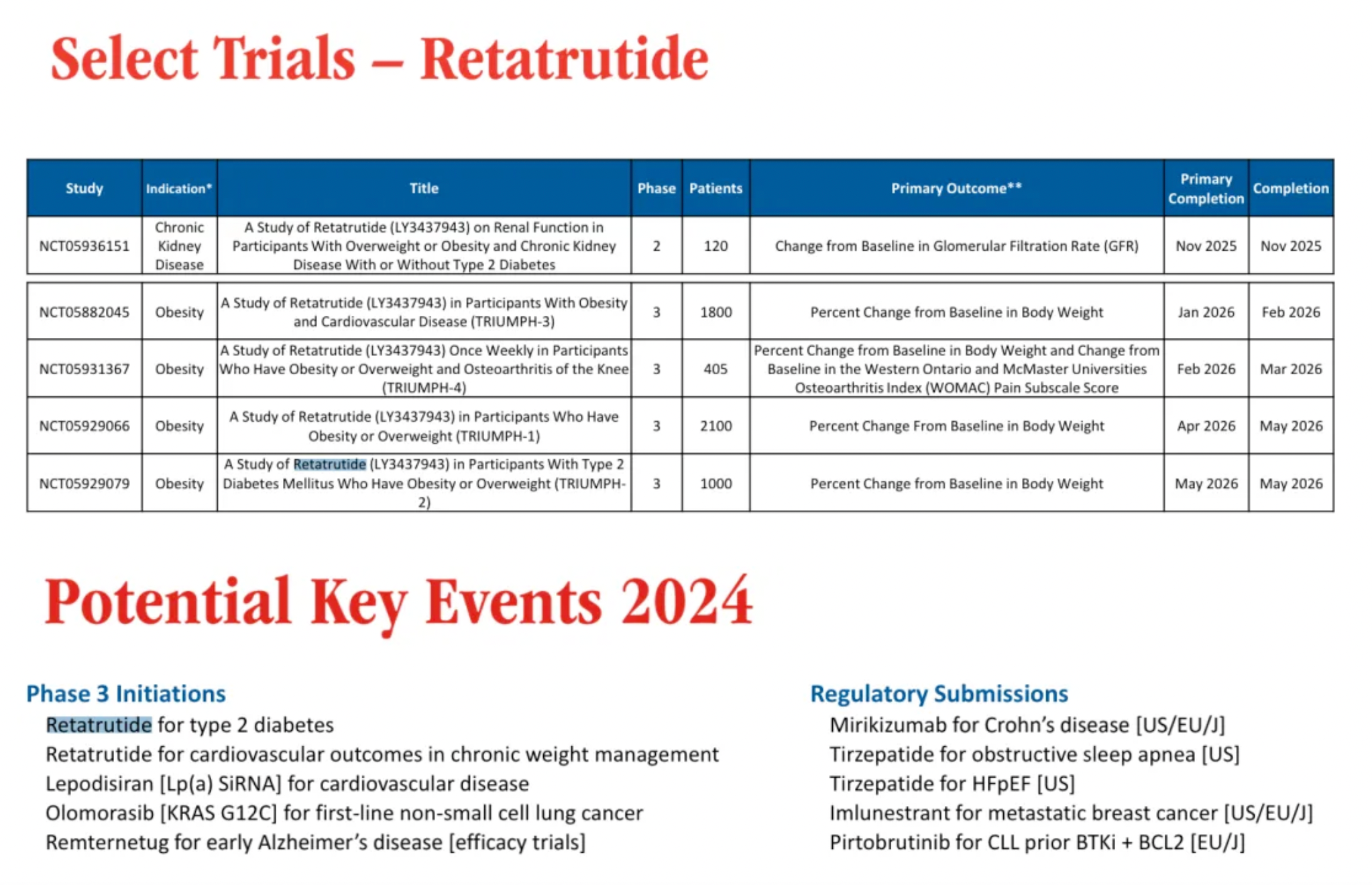
According to a clinical research plan updated by the company in early 2024, Lilly will conduct multiple Phase 3 clinical trials for Retatrutide over the next three years, with targeted indications primarily including obesity, chronic kidney disease, and type 2 diabetes.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!