Takeda Releases Phase 3 Topline Data for Soticlestat (TAK-935) in Dravet and Lennox-Gastaut Syndromes
Takeda has released the primary findings from its SKYLINE and SKYWAY clinical trials. The SKYLINE trial was a multicenter, randomized, and double-blind Phase 3 study that assessed the combination of soticlestat (TAK-935) with standard care against a placebo with standard care in patients suffering from refractory Dravet syndrome.
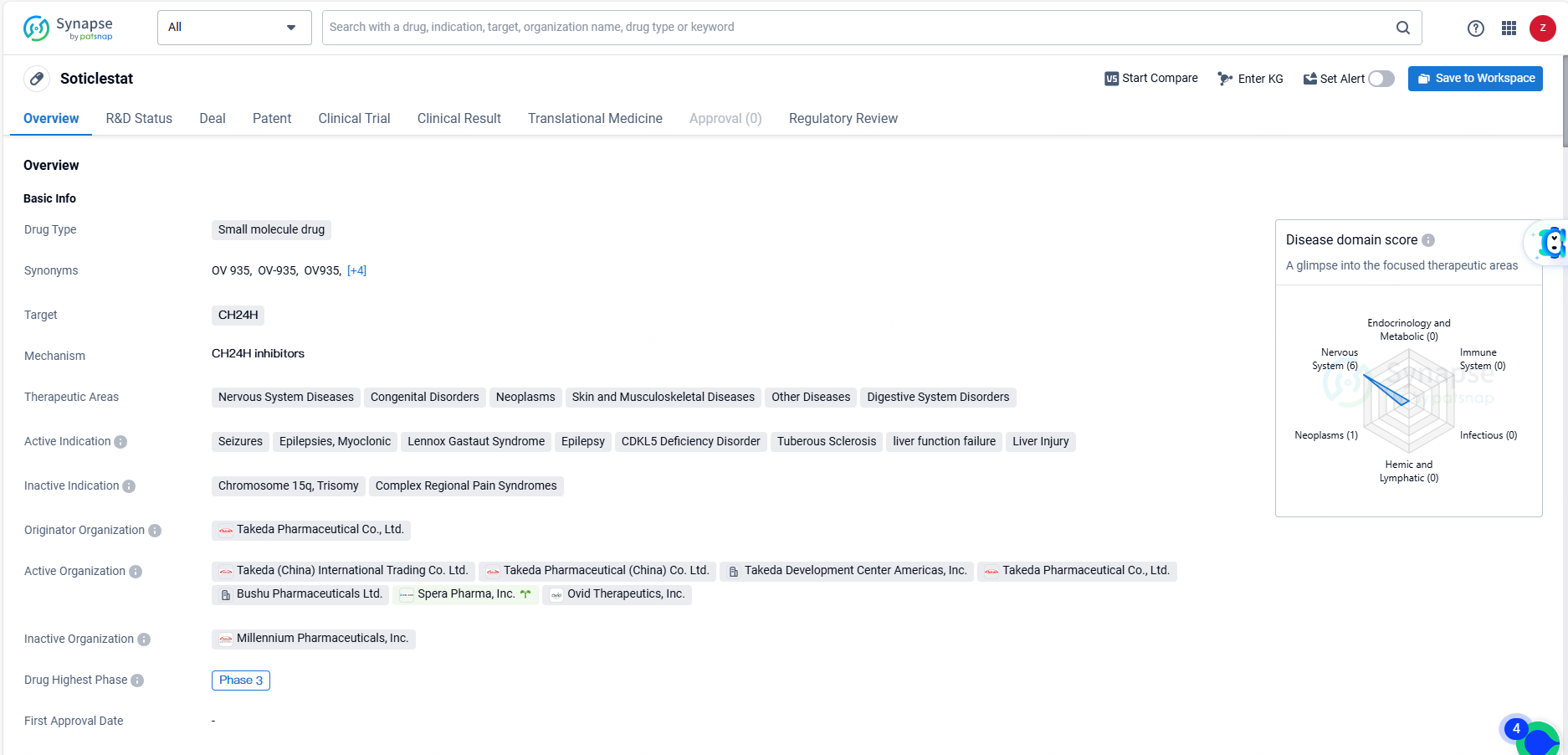
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Soticlestat did not meet the primary endpoint of reducing convulsive seizure frequency from baseline when compared to placebo. However, soticlestat exhibited clinically meaningful and nominally significant improvements in four of six key secondary endpoints, including the responder rate, caregiver and clinician global impression of improvement, and scales measuring seizure intensity and duration over a 16-week treatment period.
SKYWAY was a Phase 3, multicenter, randomized, double-blind study that compared soticlestat combined with standard care to placebo plus standard care in patients suffering from refractory Lennox-Gastaut syndrome. Soticlestat did not achieve the primary endpoint of reducing Major Motor Drop seizure frequency in comparison to placebo.
In both SKYLINE and SKYWAY studies, certain pre-specified patient subgroups showed nominally significant effects on primary and secondary efficacy endpoints, including caregiver and clinician impressions of improvement and measures of seizure intensity and duration over the 16-week treatment duration. Further detailed analyses are ongoing.
"While we hoped for more definitive outcomes on the primary endpoints, we are heartened by the positive trends observed in the overall data. We look forward to consulting with health authorities to determine the optimal path forward," stated Sarah Sheikh, M.Sc., B.M., B.Ch., MRCP, Head of the Neuroscience Therapeutic Area Unit and Global Development at Takeda.
Soticlestat (TAK-935) is an investigational, first-in-class, potent and selective inhibitor of cholesterol 24-hydroxylase, an enzyme predominantly found in the brain. This enzyme metabolizes cholesterol into 24-S hydroxycholesterol, leading to a decrease in glutamatergic hyperexcitability.
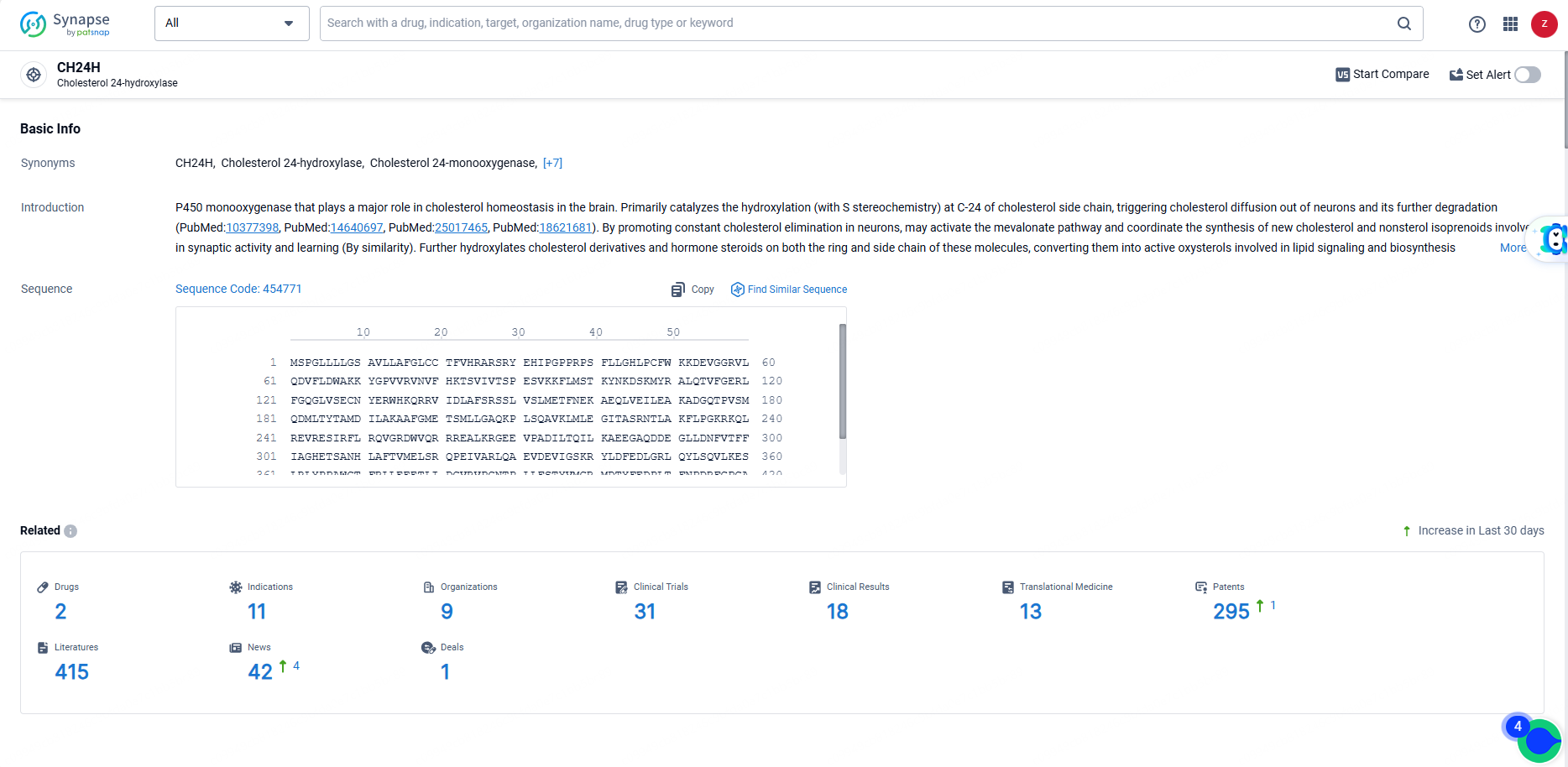
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 19, 2024, there are 2 investigational drugs for the CH24H targets, including 11 indications, 9 R&D institutions involved, with related clinical trials reaching 31, and as many as 295 patents.
Soticlestat targets CH24H and is being developed for the treatment of a wide range of therapeutic areas, including Nervous System Diseases, Congenital Disorders, Neoplasms, Skin and Musculoskeletal Diseases, Other Diseases, and Digestive System Disorders. As the drug continues its development and evaluation, it has the potential to make a meaningful impact on the treatment of various diseases and disorders, offering hope to patients and healthcare providers alike.