Nurix Therapeutics Announces Positive NX-5948 Trial Results in CLL Patients at EHA2024
Nurix Therapeutics, Inc., a biopharmaceutical firm in the clinical stage that focuses on creating targeted protein modulation therapies for cancer and inflammatory disease patients, revealed new clinical data for NX-5948 today. NX-5948 is an orally accessible degrader of Bruton’s tyrosine kinase (BTK) and is currently being tested in a Phase 1a/b clinical trial aimed at adults with recurring or treatment-resistant B-cell malignancies, such as chronic lymphocytic leukemia (CLL) and non-Hodgkin's lymphoma.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. Kim Linton, M.B.Ch.B, MRCP, Ph.D., FRCP, a senior lecturer at the University of Manchester and a consultant at The Christie NHS Foundation Trust, who is also an investigator for the clinical trial, shared the findings during an oral session at the European Hematology Association Congress held from June 13–16, 2024, in Madrid, Spain.
"The current findings from this study on advanced patients are quite remarkable at this early stage of development, and we are hopeful that NX-5948 could represent a significant advancement for patients with relapsed CLL, especially given the emerging resistance patterns to the currently available targeted therapies," stated Dr. Linton.
Additionally, Dr. Linton presented an updated case report highlighting the response of a patient with CLL and CNS involvement who had undergone three previous therapies, including treatment with a BTK inhibitor. Following daily treatment with 100 mg, and subsequently 300 mg, of NX-5948, the patient demonstrated a deepening response nearing complete response criteria by 36 weeks, with malignant cells in the cerebrospinal fluid eliminated by week 24.
The company also presented another case report of a patient who had received eleven prior therapies, including all available BTK inhibitors (ibrutinib, acalabrutinib, zanubrutinib, and pirtobrutinib). After daily administration of 200 mg of NX-5948, the patient showed a response by week 8 that continued to deepen over time, with ongoing benefits observed over more than 6 months of follow-up.
"With a growing body of positive clinical data, demonstrated activity in the CNS, and a favorable safety profile, NX-5948 is emerging as a best-in-class medicine that has the potential to provide an important treatment option for patients with CLL and NHL," stated Arthur T. Sands, M.D., Ph.D., president and CEO of Nurix. "We are planning to advance quickly with the goal of initiating pivotal trial(s) with NX-5948 in 2025."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
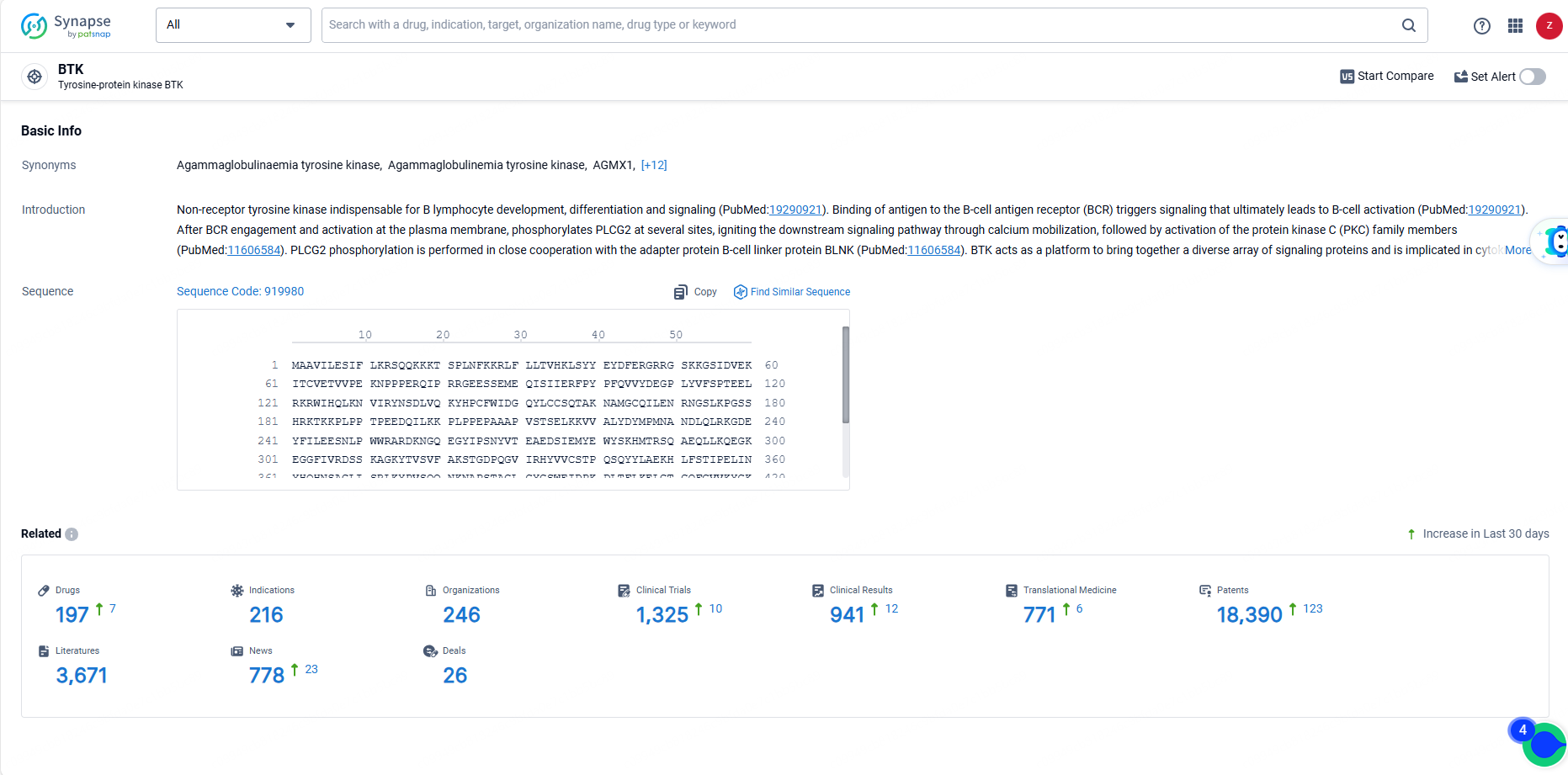
According to the data provided by the Synapse Database, As of June 19, 2024, there are 197 investigational drugs for the BTK target, including 216 indications, 246 R&D institutions involved, with related clinical trials reaching 1325, and as many as 18390 patents.
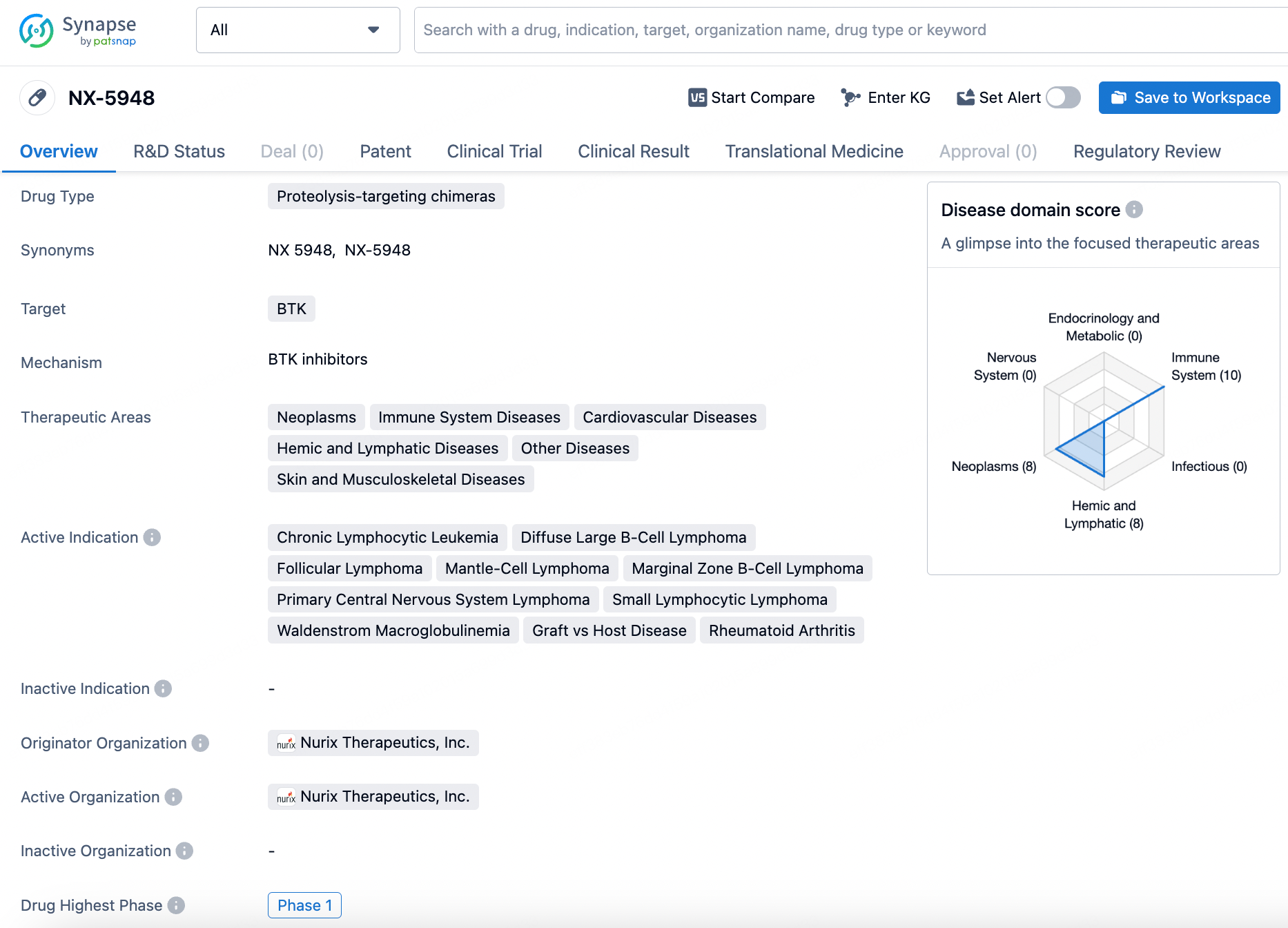
NX-5948 is a proteolysis-targeting chimera that targets the BTK protein. It is currently in the highest phase of clinical development, which is Phase 1. The drug has been designated as a Fast Track product, indicating that it is intended to treat serious conditions and fill an unmet medical need.