EMA Accepts Marketing Submission for AVT06, an Aflibercept Biosimilar to Eylea®
Alvotech (NASDAQ: ALVO), an international biotech firm focused on creating and producing biosimilar medications for global patients, and Advanz Pharma, a UK-based global pharmaceutical company concentrating on specialty, hospital, and rare disease medications in Europe, declared that the European Medicines Agency (EMA) has accepted a Marketing Authorization Application for AVT06, Alvotech’s planned biosimilar to Eylea® (aflibercept). The marketing authorization process might be finalized by the third quarter of 2025.
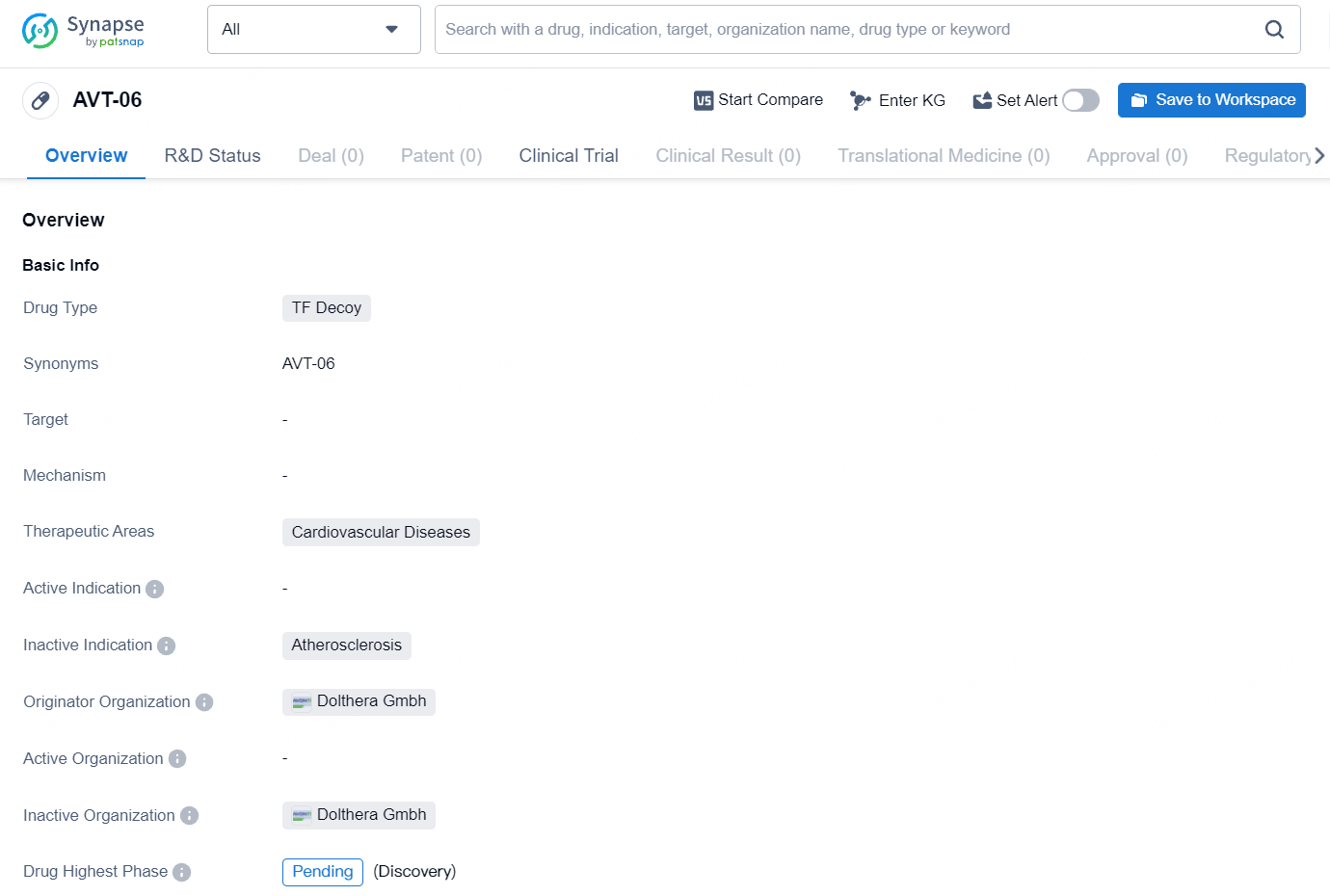
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Joseph McClellan, Chief Scientific Officer at Alvotech, commented, "Acceptance by the EMA brings us one step nearer to launching AVT06 in Europe, which is encouraging news for both patients and caregivers." He added, "The successful creation of numerous biosimilar candidates highlights Alvotech's utilization of its comprehensive biosimilars platform to enhance access to cost-effective biologic treatments."
Dr. Nick Warwick, Chief Medical Officer at Advanz Pharma, remarked, "This achievement is a significant step forward in offering patients more treatment choices and enhances Advanz Pharma’s dedication to increasing the availability of specialty, hospital, and rare disease medications throughout Europe."
Alvotech is in charge of the development and commercial supply of AVT06. Advanz Pharma handles the registration and has exclusive commercialization rights in Europe, excluding France and Germany where the rights are semi-exclusive. In France, Biogaran, a pharmaceutical company, holds semi-exclusive registration and commercialization rights. Alvotech is also working on AVT29, a biosimilar candidate for Eylea® high dose (8 mg). Both Advanz Pharma and Biogaran will commercialize AVT29 in the same countries as AVT06.
Eylea® is a commonly used biologic for eye disorder treatments, including conditions that may result in vision impairment or blindness, such as wet Age-related Macular Degeneration (AMD), macular edema, and diabetic retinopathy. In 2023, Eylea® reported sales of $2.9 billion in Europe, with global sales totaling $5.9 billion.
In January 2024, Alvotech announced favorable top-line results from a confirmatory clinical study (AVT06-GL-C01), which assessed the efficacy, safety, and immunogenicity of AVT06 compared to Eylea in patients with neovascular (wet) AMD. The study achieved its primary endpoint, demonstrating therapeutic equivalence and similar safety and immunogenicity profiles between Alvotech’s biosimilar candidate and Eylea.
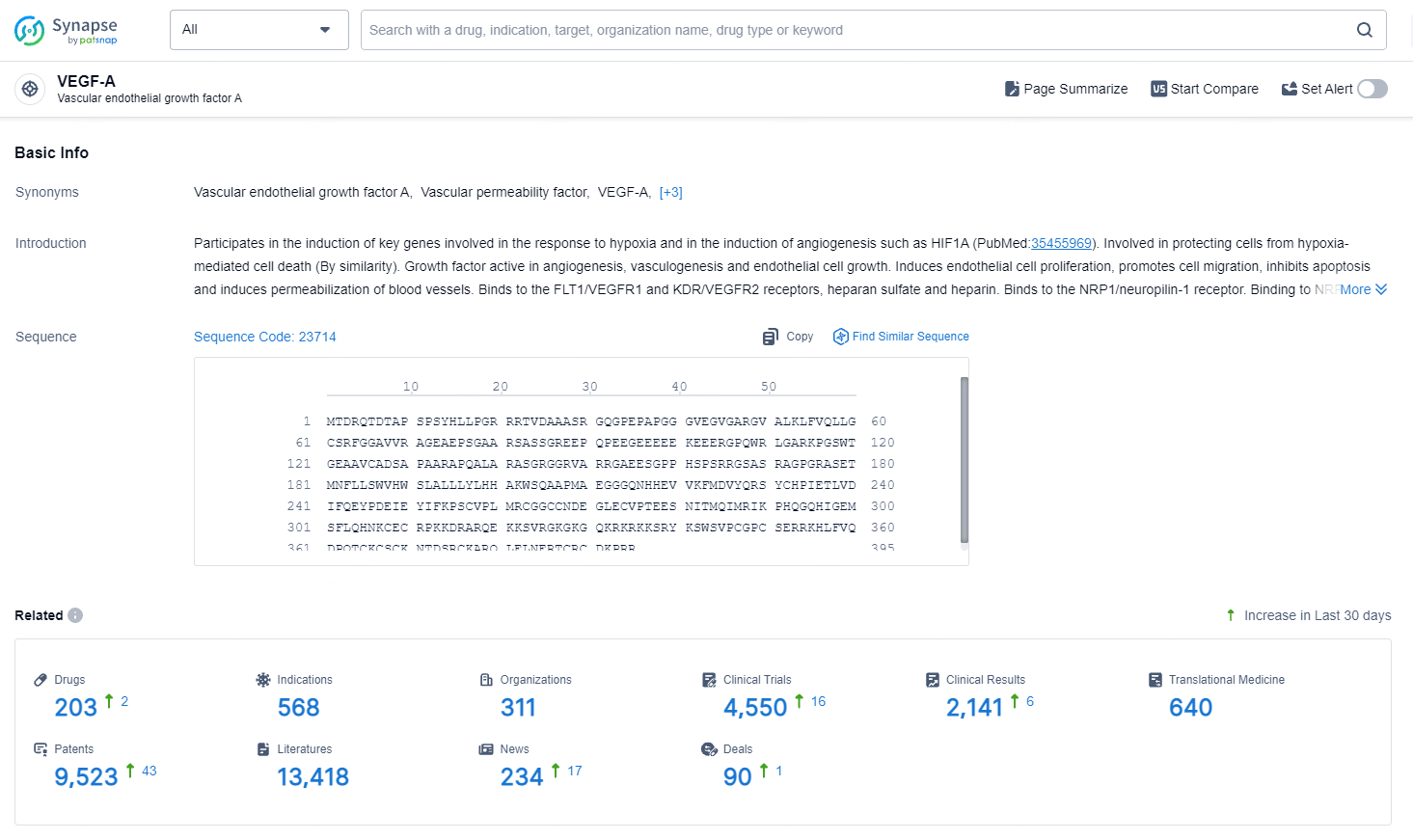
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 19, 2024, there are 203 investigational drug for the VEGF-A target, including 568 indications, 311 R&D institutions involved, with related clinical trials reaching 4550, and as many as 9523 patents.
AVT06/AVT29 is a recombinant fusion protein and a biosimilar candidate to Eylea® (aflibercept) 2 mg and 8 mg dose, which binds vascular endothelial growth factors (VEGF), inhibiting the binding and activation of VEGF receptors, neovascularization, and vascular permeability. AVT06/AVT29 are investigational products and have not received regulatory approval in any country. Biosimilarity has not been established by regulatory authorities and is not claimed.