EMA Validates Bristol Myers Squibb's Application for Opdivo and Yervoy in Advanced Liver Cancer
Bristol Myers Squibb revealed that the European Medicines Agency has acknowledged its Type II variation application for Opdivo® (nivolumab) in combination with Yervoy® (ipilimumab) as a possible first-line therapeutic option for adult individuals with unresectable or advanced hepatocellular carcinoma who have not undergone previous systemic treatment. The submission was supported by data from the Phase 3 CheckMate -9DW study. This validation indicates that the application is comprehensive and starts the centralized procedure review process by the EMA.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
 Dr. Dana Walker, M.D., M.S.C.E., vice president, global program lead for gastrointestinal and genitourinary cancers at Bristol Myers Squibb, mentioned that liver cancer diagnoses in the European Union reach about 62,000 cases per year, with hepatocellular carcinoma (HCC) being the most common. Despite recent advancements in treatments, patients in advanced stages still face poor prognoses, emphasizing the critical need for therapies that provide better clinical outcomes.
Dr. Dana Walker, M.D., M.S.C.E., vice president, global program lead for gastrointestinal and genitourinary cancers at Bristol Myers Squibb, mentioned that liver cancer diagnoses in the European Union reach about 62,000 cases per year, with hepatocellular carcinoma (HCC) being the most common. Despite recent advancements in treatments, patients in advanced stages still face poor prognoses, emphasizing the critical need for therapies that provide better clinical outcomes.
"We are eager to collaborate with the European Medicines Agency (EMA) to advance our application for the combination of Opdivo and Yervoy as a new first-line dual immunotherapy treatment option for adults with unresectable or advanced HCC in the European Union," Dr. Walker added.
In the Phase 3 CheckMate -9DW trial, the combination of Opdivo and Yervoy showed significant improvements in overall survival when compared to lenvatinib or sorafenib, demonstrating the clinical benefits of this first-line treatment. The safety profile for Opdivo plus Yervoy was consistent with previously reported data and manageable using established protocols, with no new safety signals detected. These results were presented at the 2024 Annual Meeting of the American Society of Clinical Oncology.
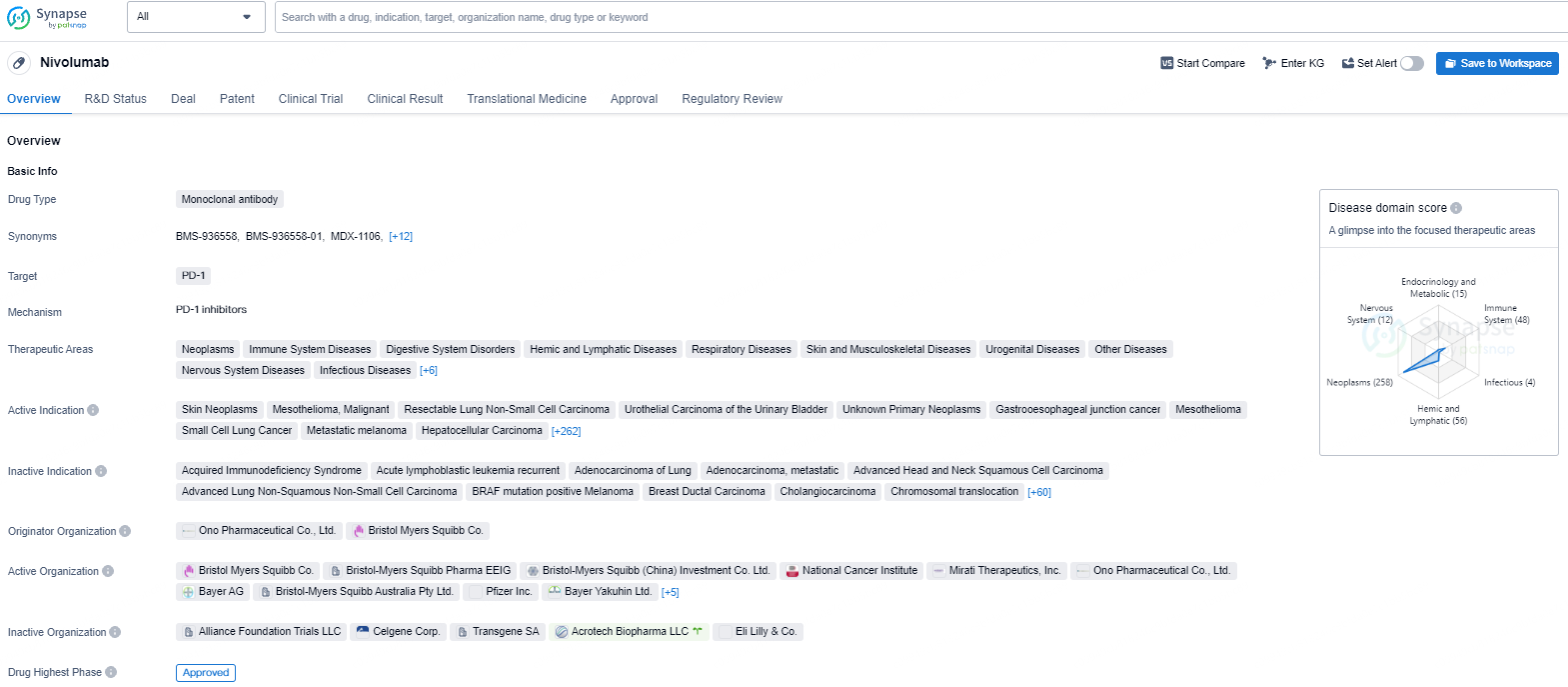
Opdivo, a programmed death-1 (PD-1) immune checkpoint inhibitor, is designed to utilize the body's immune system to restore anti-tumor responses. By engaging the immune system to combat cancer, Opdivo has become a crucial treatment across multiple cancer types.
Bristol Myers Squibb's global development program for Opdivo leverages their expertise in Immuno-Oncology, encompassing extensive clinical trials across various phases and tumor types, including Phase 3 trials. To date, over 35,000 patients have participated in Opdivo clinical trials. These studies have also contributed to understanding the role of biomarkers in patient care and how patients with different PD-L1 expressions may benefit from Opdivo.
Yervoy is a human monoclonal antibody that targets cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). The U.S. Food and Drug Administration approved Yervoy for 3 mg/kg monotherapy in patients with unresectable or metastatic melanoma on March 25, 2011. It is approved for the same condition in over 50 countries, and there is an extensive ongoing development program for Yervoy covering multiple tumor types.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
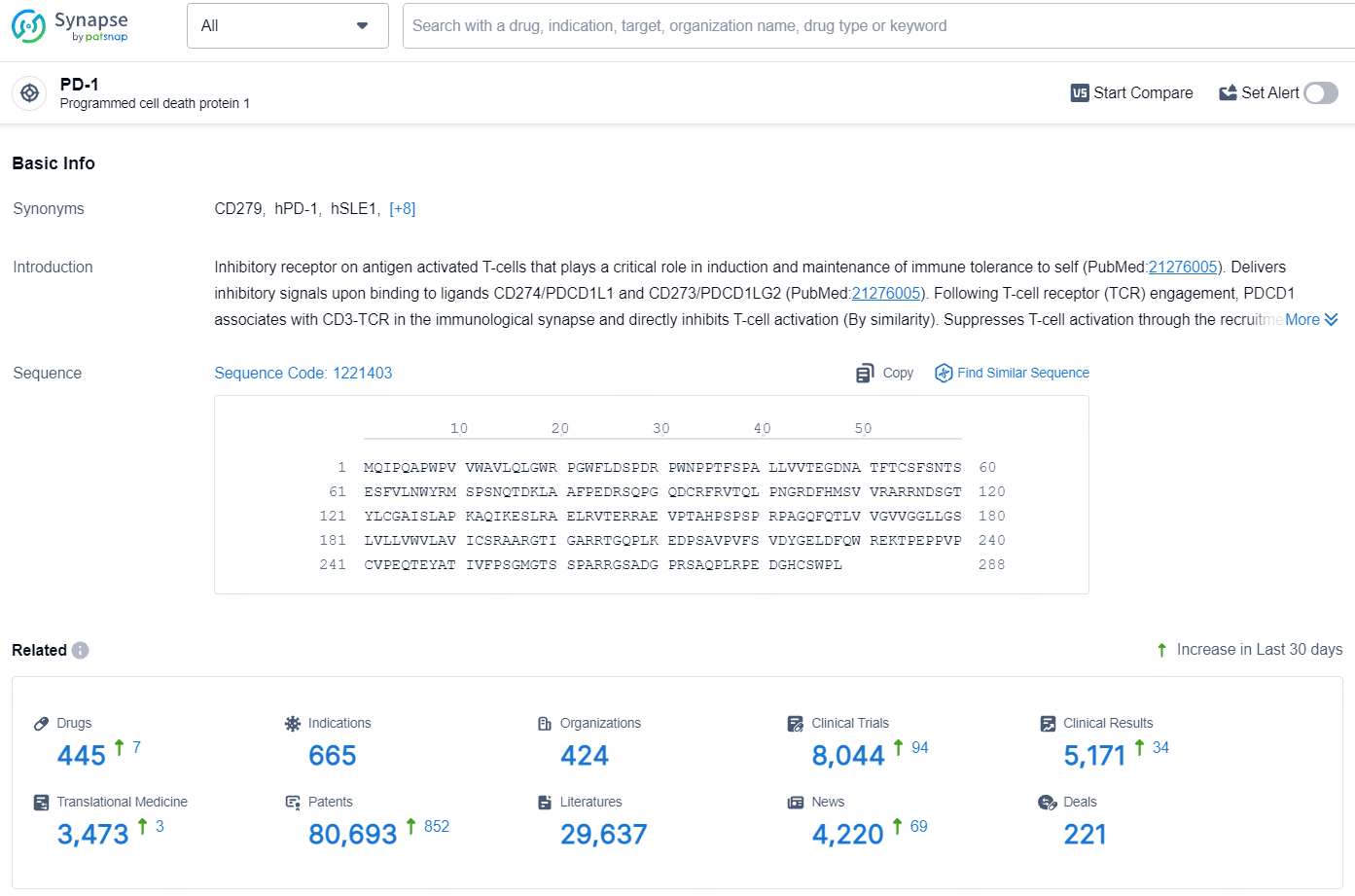
According to the data provided by the Synapse Database, As of July 24, 2024, there are 445 investigational drugs for the PD-1 target, including 665 indications, 424 R&D institutions involved, with related clinical trials reaching 8044, and as many as 80693 patents.
Opdivo was the first PD-1 immune checkpoint inhibitor to receive regulatory approval anywhere in the world. Opdivo is currently approved in more than 65 countries, including the United States, the European Union, Japan and China. In October 2015, the Company’s Opdivo and Yervoy combination regimen was the first Immuno-Oncology combination to receive regulatory approval for the treatment of metastatic melanoma and is currently approved in more than 50 countries, including the United States and the European Union.