Encouraging Results for Ractigen's RAG-17 in ALS-SOD1 Therapy from Independent Study
Ractigen Therapeutics, a pharmaceutical firm focusing on clinical-stage innovations, has reported favorable clinical findings from an Investigator-Initiated Trial (IIT) involving RAG-17, a small interfering RNA (siRNA) aimed at the Superoxide Dismutase 1 (SOD1) gene. The trial demonstrated positive outcomes in addressing amyotrophic lateral sclerosis (ALS) linked to SOD1 mutations (ALS-SOD1).
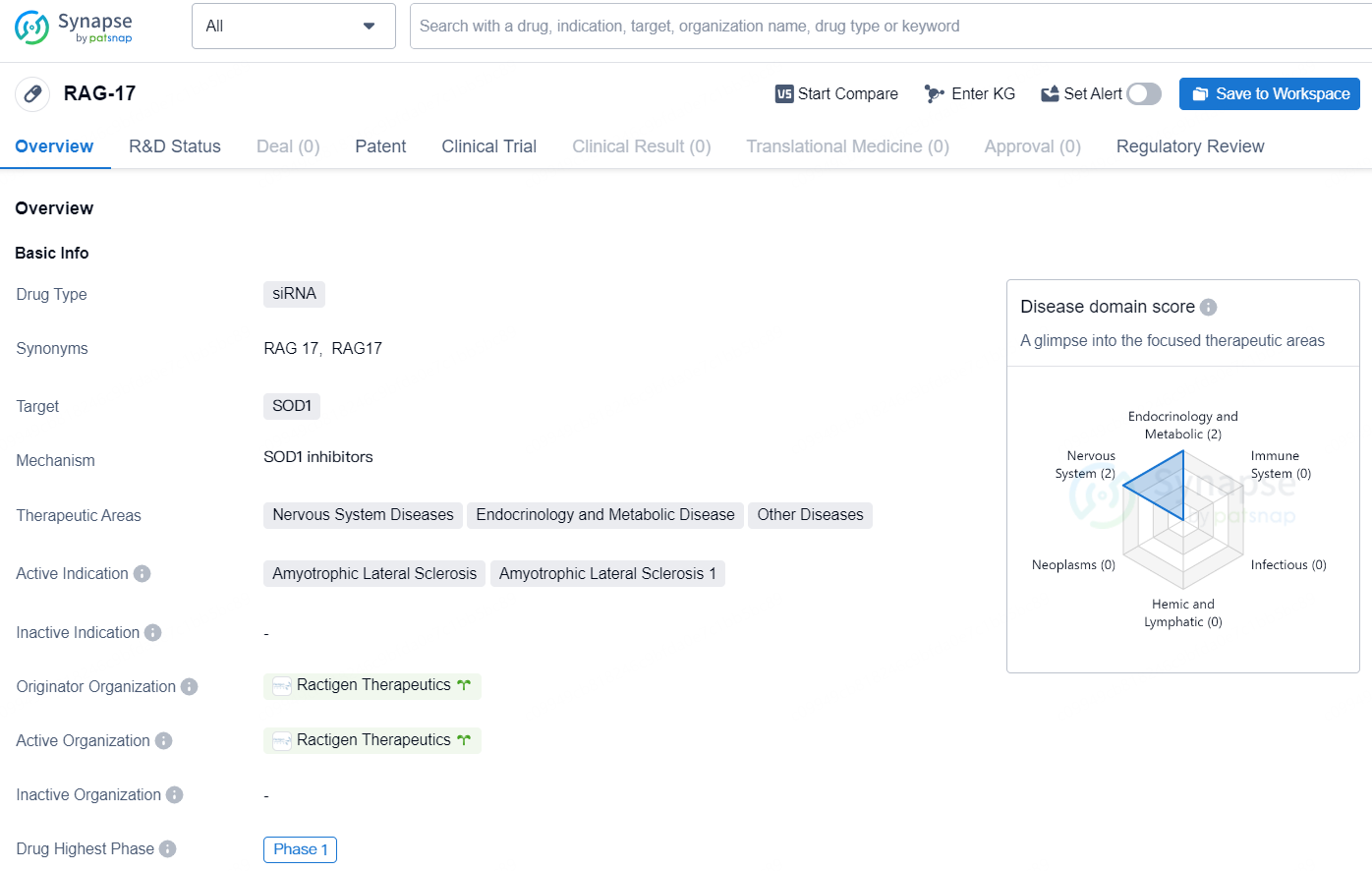
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Dr. Yilong Wang spearheaded a clinical study at Beijing Tiantan Hospital, a premier institution in China specializing in neurological conditions. The study involved six ALS-SOD1 patients and mainly focused on evaluating the safety profile of RAG-17. Administering RAG-17 intrathecally showed good tolerance at all dosage levels, with adverse events being mild in nature. Extensive safety assessments, including laboratory tests, vital sign monitoring, and electrocardiograms, further confirmed its favorable safety characteristics.
Additionally, preliminary signs indicated potential clinical benefits. Changes in clinical outcomes and key biomarkers suggested that RAG-17 could be effective for this patient group. These clinical findings are consistent with comprehensive preclinical data from Ractigen, which showed significant therapeutic benefits of RAG-17 in SOD1-G93A ALS mouse and rat models, such as delayed disease progression and extended survival.
"The initial clinical data is particularly encouraging and represents a step forward in our mission to bring new hope to ALS patients," noted Dr. Long-Cheng Li, Founder and CEO of Ractigen Therapeutics. "The positive results from this trial highlight RAG-17's potential as a disease-modifying therapy for ALS-SOD1. We remain fully committed to advancing its clinical development and ultimately providing this critical therapy to patients."
The promising data will be showcased at three upcoming conferences: the 27th National Conference of Neurology in China in September, Neuroscience 2024 in Chicago, USA, in October, and the 35th International Symposium on ALS/MND in Montreal, Canada, in December—one of the major annual conferences for ALS and motor neuron disease research.
In March 2023, RAG-17 was granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA), and its Investigational New Drug (IND) application for clinical trials in the U.S. was approved. Furthermore, in May 2024, the IND application received approval from the Center for Drug Evaluation (CDE) under China National Medical Products Administration (NMPA) for clinical trials in China.
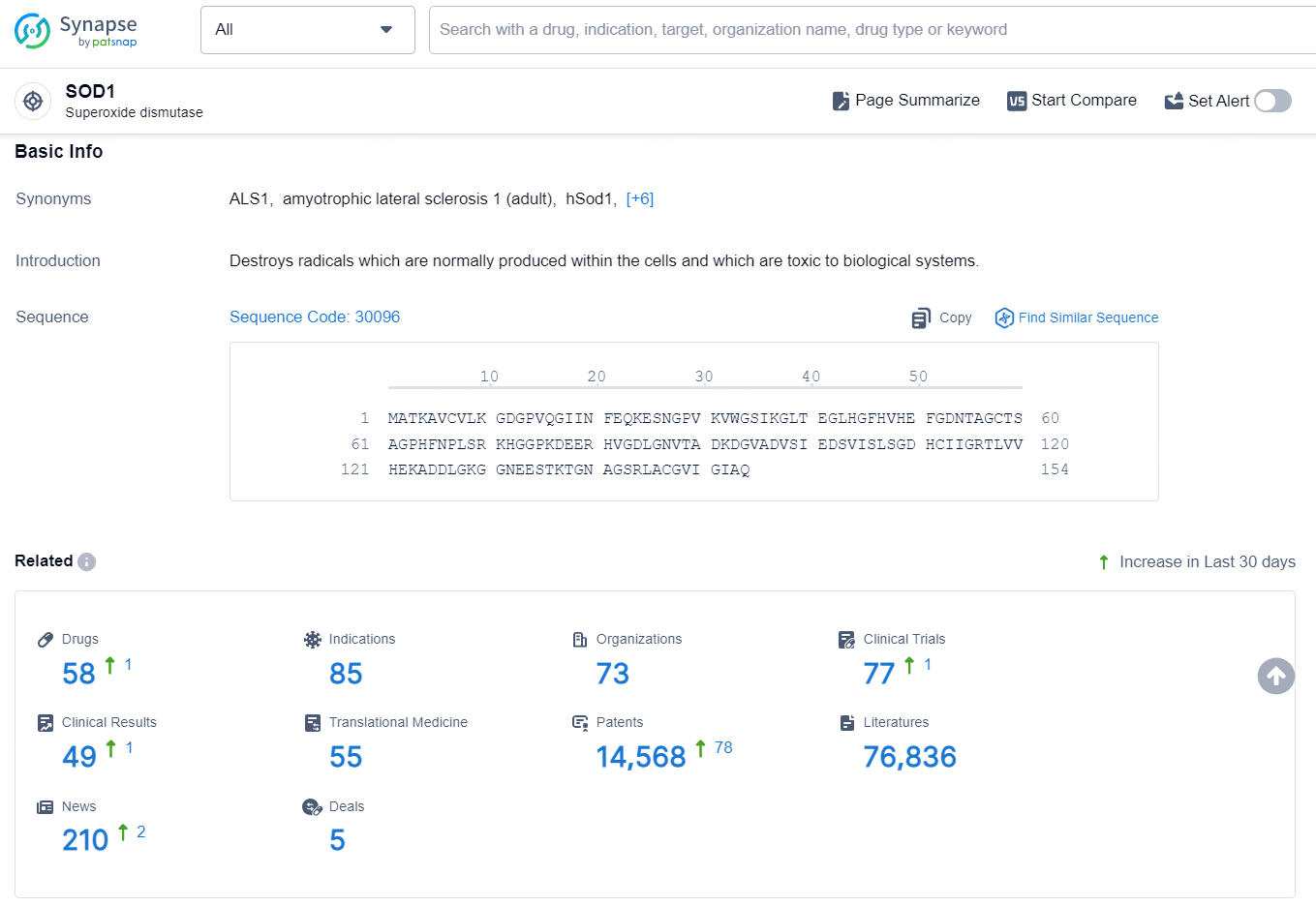
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 58 investigational drug for the SOD1 targets, including 85 indications, 73 R&D institutions involved, with related clinical trials reaching 77, and as many as 14568 patents.
RAG-17 is a siRNA drug that targets the SOD1 gene and is being developed by Ractigen Therapeutics. The drug is indicated for the treatment of Amyotrophic Lateral Sclerosis (ALS) and has reached Phase 1 in both global and Chinese regulatory processes. Additionally, [RAG-17] is classified as an Orphan Drug, which provides regulatory and financial incentives for the development of drugs that target rare diseases.