Latest Results on HER2-Mutant NSCLC Drug BAY 2927088 Revealed at WCLC
Bayer revealed promising outcomes from the expansion phase of the ongoing Phase I/II SOHO-01 trial assessing the safety and early efficacy of BAY 2927088 in patients with advanced HER2-mutant non-small cell lung cancer (NSCLC). The findings were shared at the Presidential Symposium of the IASLC 2024 World Conference on Lung Cancer (WCLC), organized by the International Association for the Study of Lung Cancer, held in San Diego, United States, between September 7-10, 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Christian Rommel, Ph.D., Head of Research and Development at Bayer’s Pharmaceuticals Division, stated, “The promising outcomes witnessed in the expansion phase of the SOHO-01 trial highlight the potential of this targeted therapy to enhance results for patients with HER2-mutant NSCLC. This specific lung cancer subtype has limited treatment alternatives and typically forecasts a poor prognosis.” He added, “Developing BAY 2927088 reflects our persistent dedication to lung cancer research and our relentless pursuit of creating precise, individualized healthcare solutions for the most critically underserved diseases.”
Xiuning Le, MD, PhD, lead investigator of SOHO-01 from The University of Texas MD Anderson Cancer Center, Houston, TX, remarked, “HER2-mutant NSCLC is a demanding disease that can mainly impact young individuals and lifetime non-smokers. With the current absence of effective treatments, there’s a significant need for therapies that are both efficacious and manageable.” She continued, “BAY 2927088 demonstrated preliminary efficacy in both response rate and duration alongside a manageable safety profile, underscoring the necessity for ongoing innovation to improve care standards.”
The focus of the study's expansion phase was to evaluate the safety, tolerability, and pharmacokinetics of BAY 2927088 in patients with HER2-mutant NSCLC. The findings reinforce the continuous examination of BAY 2927088 in patients with advanced NSCLC harboring HER2 mutations. Recently, Bayer disclosed the enrollment of the first patient in the international Phase III SOHO-02 trial, a multicenter, open-label, randomized clinical trial aimed at evaluating the efficacy and safety of the investigational agent BAY 2927088 as a first-line treatment for patients with advanced NSCLC characterized by activating HER2 mutations.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
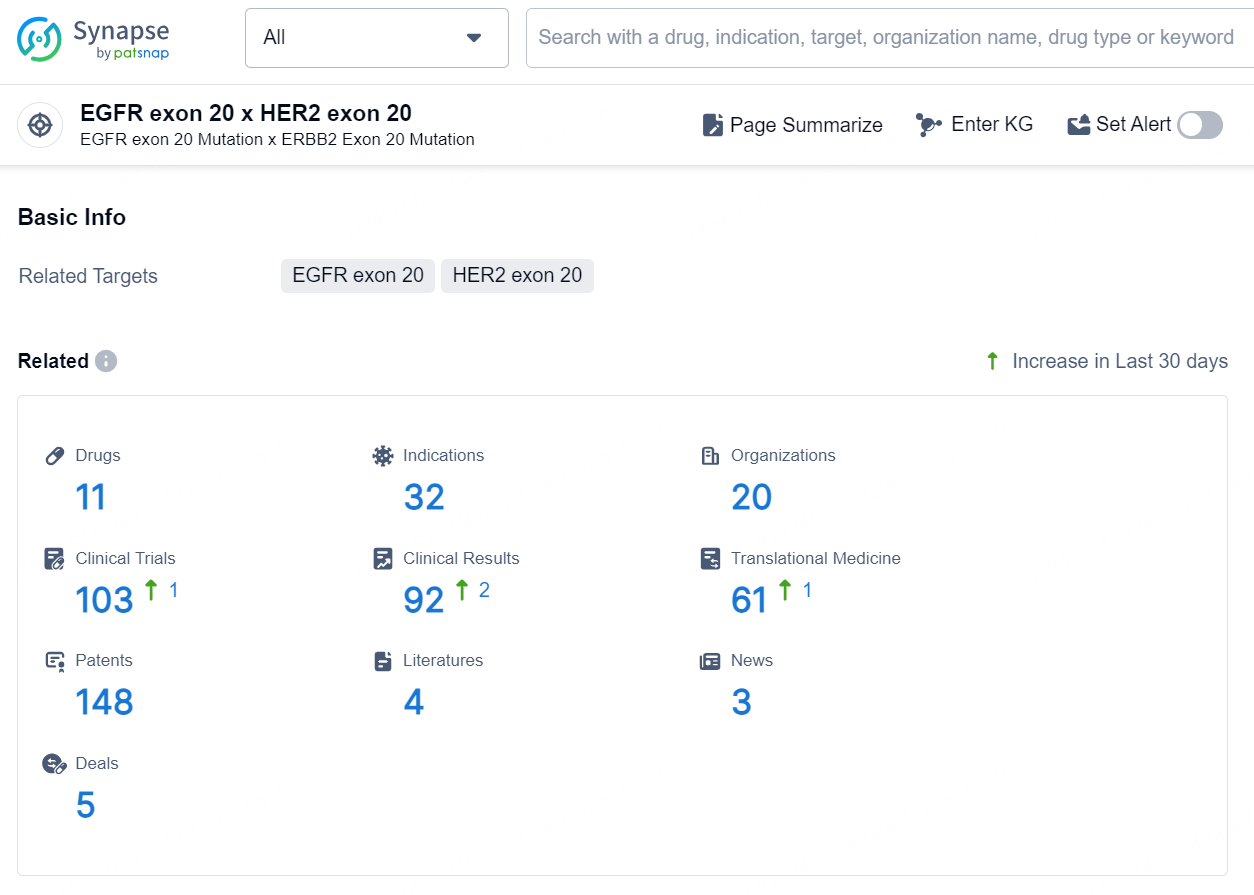
According to the data provided by the Synapse Database, As of September 12, 2024, there are 11 investigational drugs for the EGFR exon 20 and HER2 exon 20 target, including 32 indications, 20 R&D institutions involved, with related clinical trials reaching 103, and as many as 148 patents.
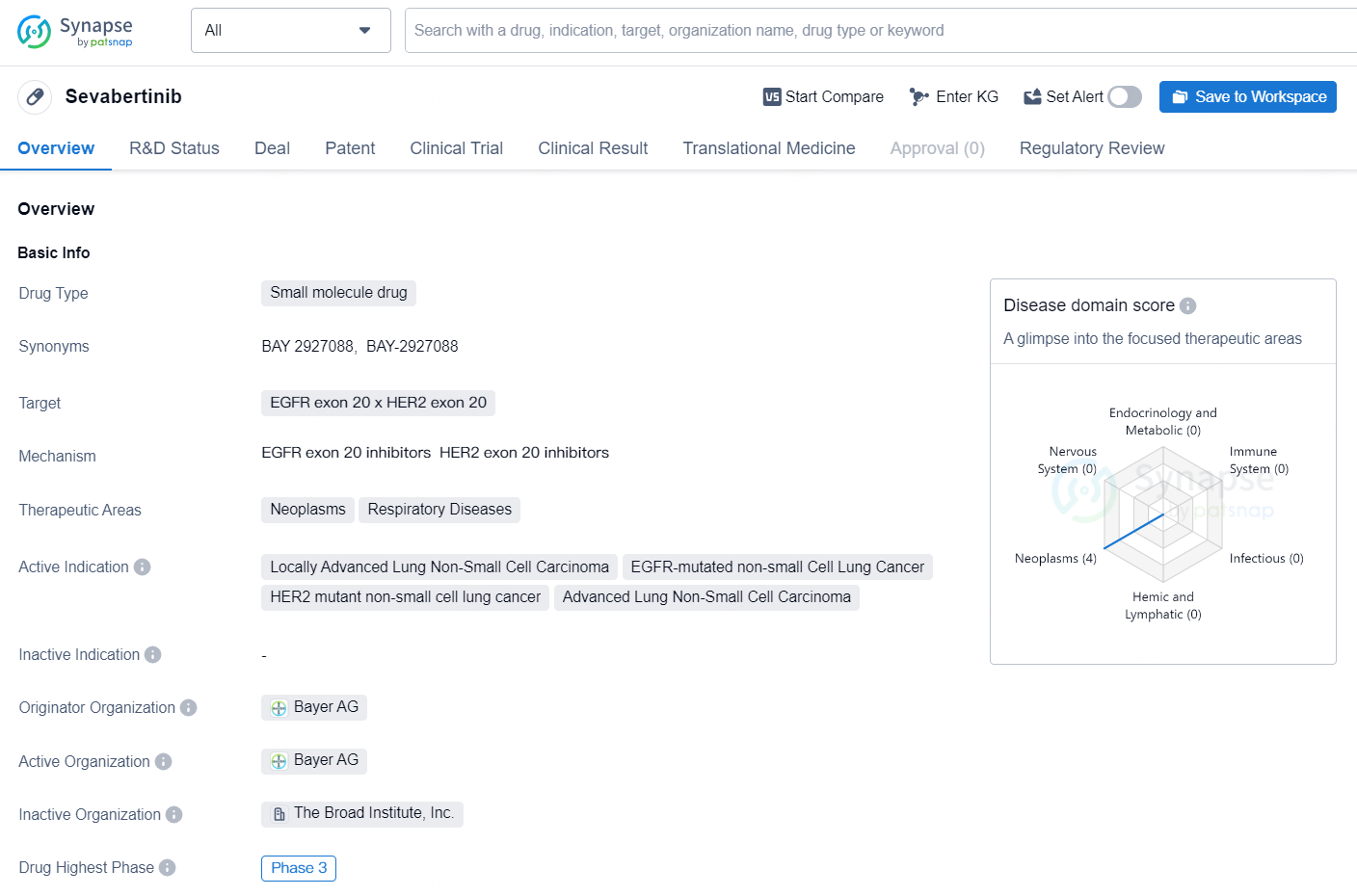
Sevabertinib is a small molecule drug that targets the EGFR exon 20 and HER2 exon 20. It falls within the therapeutic areas of neoplasms and respiratory diseases, with active indications including locally advanced lung non-small cell carcinoma, EGFR-mutated non-small cell lung cancer, HER2 mutant non-small cell lung cancer, and advanced lung non-small cell carcinoma. The drug is being developed by Bayer AG and has reached the highest global phase of Phase 3. In China, Sevabertinib has also reached Phase 3.