Datopotamab Deruxtecan Shows 14.6-Month Median Survival in Phase 3 TROPION-Lung01 Trial for Advanced Nonsquamous NSCLC
Extensive findings from the TROPION-Lung01 phase 3 study indicated a clinically significant tendency towards enhancing overall survival (OS) with datopotamab deruxtecan (Dato-DXd) in contrast to docetaxel, the prevailing standard chemotherapy, in adult individuals with locally advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC) who had undergone at least one previous line of treatment.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
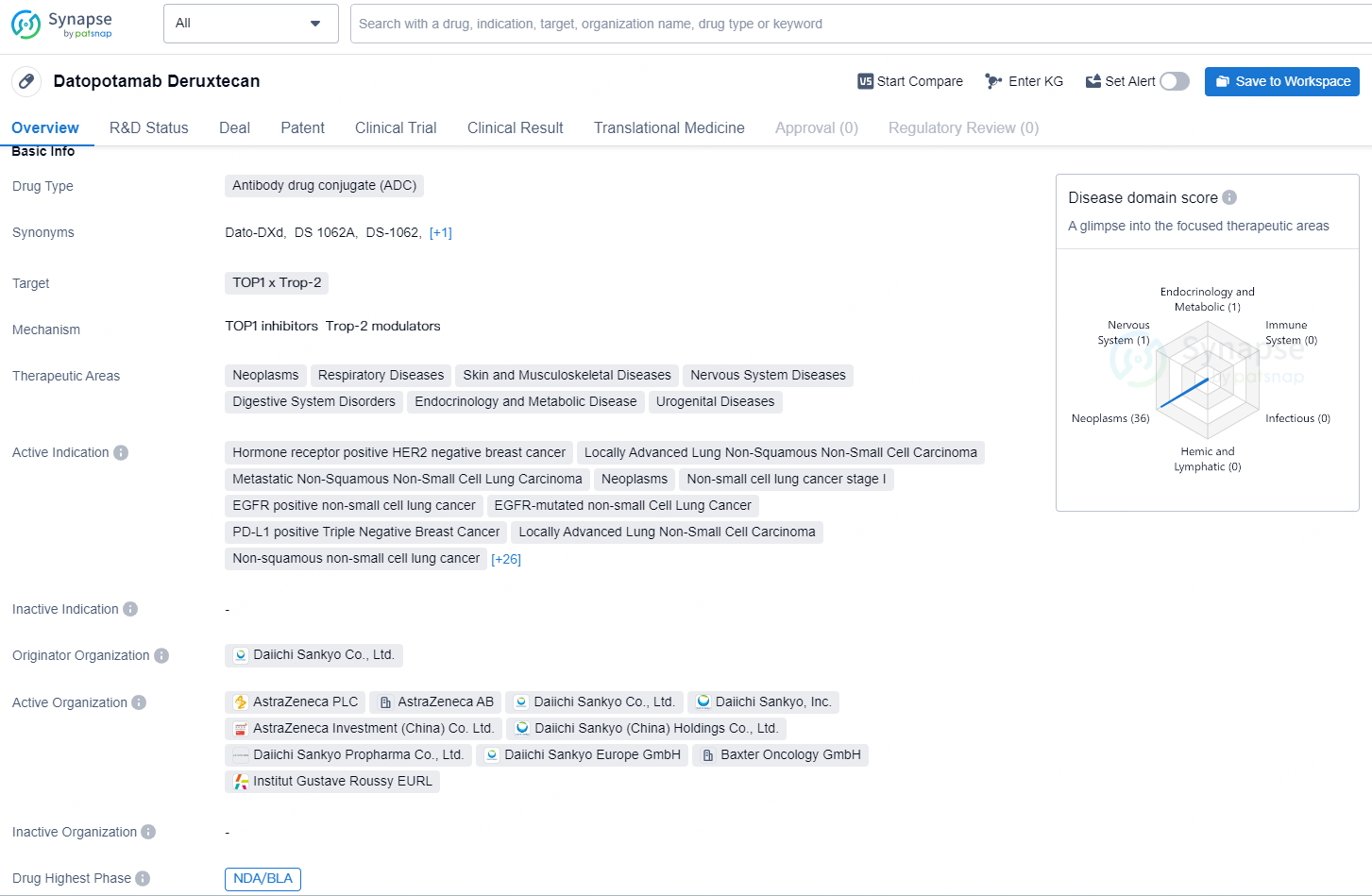
Datopotamab deruxtecan, an engineered TROP2-targeted DXd antibody-drug conjugate (ADC) developed by Daiichi Sankyo (TSE: 4568), is being co-developed by Daiichi Sankyo and AstraZeneca (LSE/STO/Nasdaq: AZN).
In the overall trial cohort, the OS results numerically favored datopotamab deruxtecan over docetaxel (median OS 12.9 months vs. 11.8 months), although this was not statistically significant (hazard ratio [HR] = 0.94; 95% confidence interval [CI]: 0.78-1.14; p=0.530). In the predefined subgroup of patients with nonsquamous NSCLC, datopotamab deruxtecan offered a 2.3-month increase in OS compared to docetaxel (14.6 months vs. 12.3 months; HR = 0.84; 95% CI: 0.68-1.05). Furthermore, OS improvement was seen in nonsquamous NSCLC patients regardless of actionable genomic alterations. However, in squamous NSCLC patients, datopotamab deruxtecan did not demonstrate an improvement in OS, consistent with prior analyses.
"Despite various attempts to outdo docetaxel with new treatments in previously treated advanced or metastatic non-small cell lung cancer, survival remains around one year," commented Jacob Sands, MD, from the Dana-Farber Cancer Institute and trial investigator. "For datopotamab deruxtecan to show a statistically significant progression-free survival improvement along with better response rates, increased duration of response, and an OS improvement that aligns numerically with the progression-free survival, is clinically meaningful for patients with nonsquamous lung cancer."
The safety profile of datopotamab deruxtecan in the TROPION-Lung01 trial was consistent with earlier data, showing lower rates of dose reduction (20% vs. 30%) and treatment discontinuation (8% vs. 12%) due to adverse events compared with docetaxel. Median treatment duration was 4.2 months for datopotamab deruxtecan versus 2.8 months for docetaxel. Grade 3 or higher treatment-related adverse events (TRAE) were reported in 26% of the datopotamab deruxtecan group and 42% of the docetaxel group. The most common Grade 3 or higher TRAEs for datopotamab deruxtecan in comparison to docetaxel included neutropenia (1% vs. 23%), leukopenia (0% vs. 13%), stomatitis (7% vs. 1%), anemia (4% vs. 4%), interstitial lung disease (ILD) (4% vs. 1%), and asthenia (3% vs. 2%). No new ILD events of any grade were adjudicated as drug-related since prior analysis.
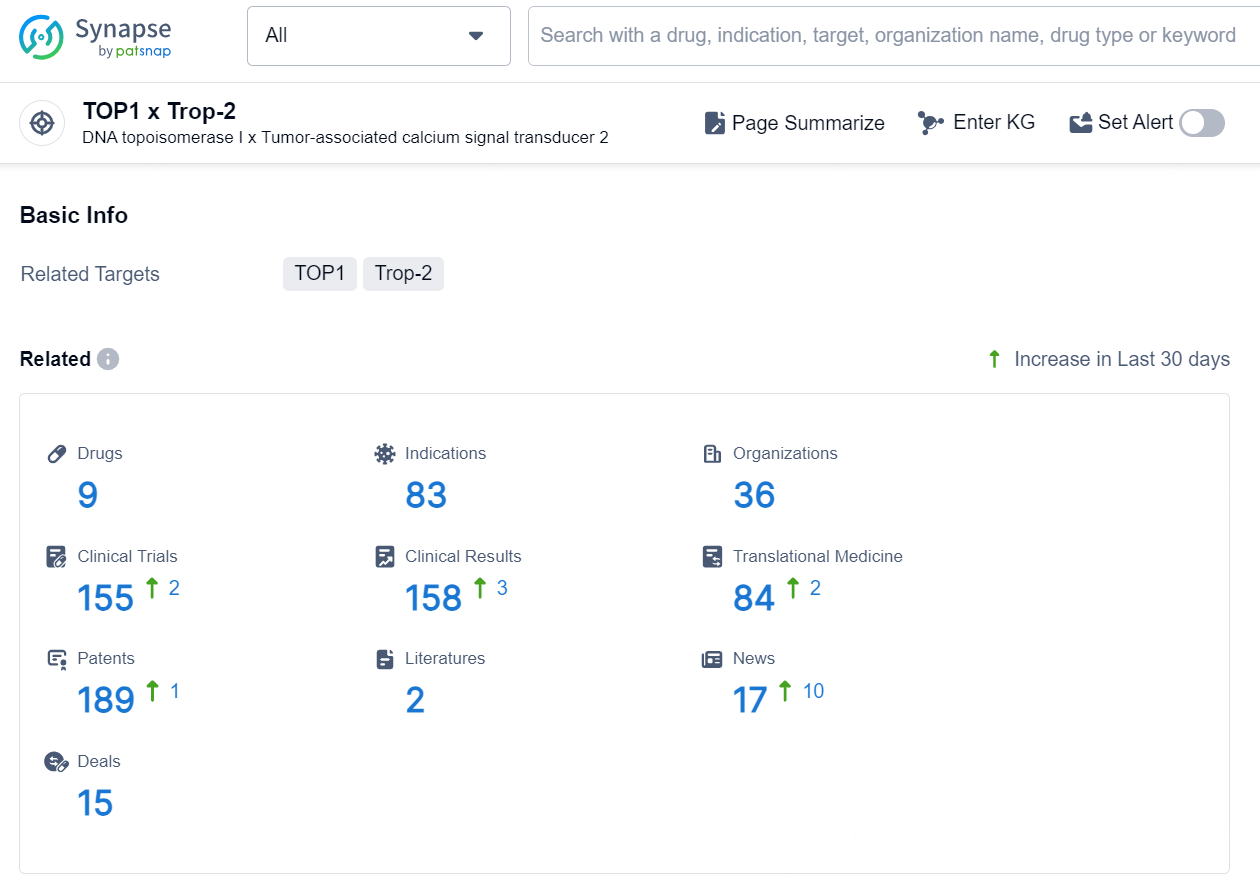
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 9 investigational drugs for the TOP1 and Trop-2 target, including 83 indications, 36 R&D institutions involved, with related clinical trials reaching 155, and as many as 189 patents.
Datopotamab deruxtecan is an antibody drug conjugate (ADC) that targets TOP1 and Trop-2. It has a wide range of therapeutic areas, including neoplasms, respiratory diseases, skin and musculoskeletal diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, and urogenital diseases.