Essential Tool in Interventional Radiology: Contrast Agent
A Contrast agent, also known as a contrast medium, is a specific substance that is introduced into the human body for the purpose of medical imaging – it is used to alter the contrast of images of parts of the body. Contrast agents are one of the most frequently used drugs in interventional radiology procedures, primarily for displaying blood vessels and body cavities. There are various types of contrast agents, with iodine-based agents commonly used in interventional radiology. The development and improvement of contrast agents have evolved in parallel with that of interventional radiology, ever since the first successful femoral artery imaging was carried out in the United States in 1924 using a 50% sodium iodide solution.
In the era of the medical imaging market, portable ultrasound possesses a strong appeal. One of the obstacles in the development of ultrasound is the issue of contrast agents. Contrast agents can greatly enhance image quality and diagnostic results. For some diagnoses, they will be one of the key factors helping ultrasound to effectively compete with MRI and CT. Contrast agents are often used in MRI and CT scans, with MRI using Superparamagnetic Iron Oxide (SPIO), but there are few contrast agents available for ultrasound. Even among these few contrast agents, some applications are controversial. The US FDA and the European Medicines Evaluation Agency have issued warnings on the use of these contrast agents in some heart diseases. Medical experts are working hard to lobby against these rulings. If these market regulatory obstacles are cleared, it will have a significant impact on the growth of the ultrasound and ultrasound contrast agent market.
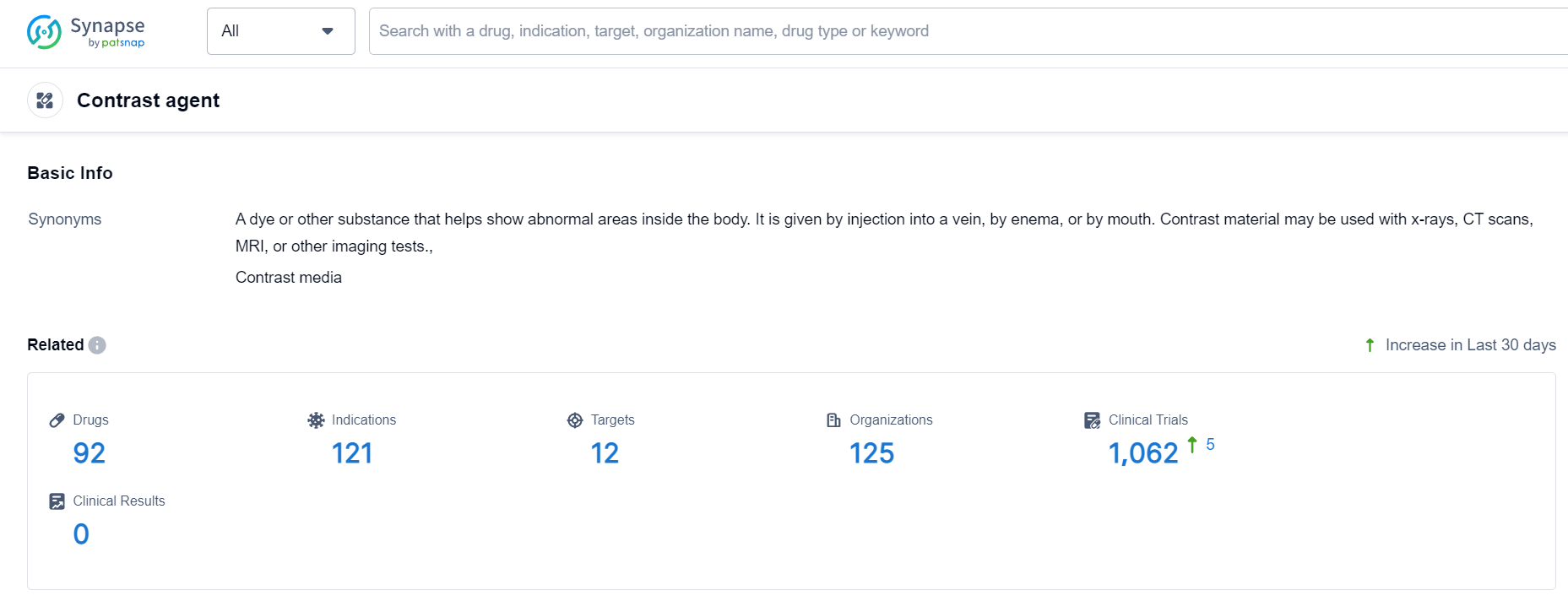
Contrast Agent Competitive Landscape
According to Patsnap Synapse, as of 8 Oct 2023, there are a total of 92 Contrast agent drugs worldwide, from 125 organizations, covering 12 targets, 121 indications, and conducting 1062 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
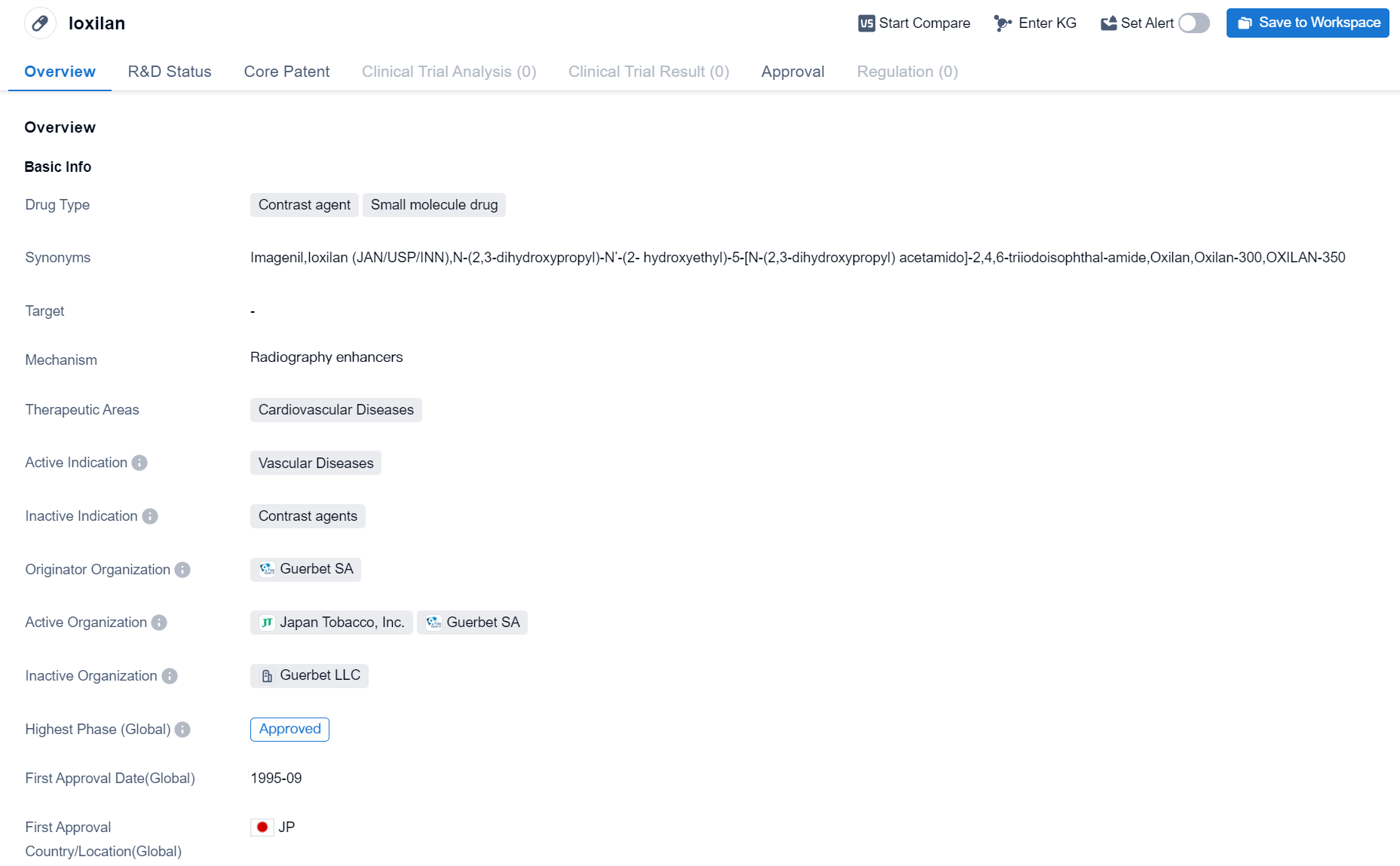
Approved Contrast Agent Medicinal: Ioxilan
Ioxilan is a contrast agent and small molecule drug used in the field of biomedicine. It is primarily indicated for the treatment of cardiovascular diseases, specifically vascular diseases. The drug was first approved in Japan in September 1995.
Ioxilan is developed by Guerbet SA, an originator organization in the pharmaceutical industry. As a contrast agent, it is commonly used in medical imaging procedures to enhance the visibility of blood vessels and organs during diagnostic tests such as angiography and computed tomography (CT) scans. By improving the contrast between blood vessels and surrounding tissues, Ioxilan helps healthcare professionals to accurately diagnose and monitor various cardiovascular conditions.
Being a small molecule drug, Ioxilan is composed of low molecular weight compounds that can easily penetrate cell membranes and interact with specific targets in the body. This characteristic allows for efficient delivery and distribution of the drug throughout the cardiovascular system, ensuring its effectiveness in diagnosing and treating vascular diseases.
The highest phase of development for Ioxilan is approved, indicating that it has successfully completed clinical trials and regulatory processes. This approval status signifies that the drug has met the necessary requirements and can be marketed and prescribed for patients.
Since its first approval in Japan in 1995, Ioxilan has likely gained recognition and acceptance in the medical community for its diagnostic capabilities in cardiovascular diseases. It is important to note that while the provided information is factual, further research and analysis may be required to fully understand the drug's market presence, competitive landscape, and potential future developments.
In summary, Ioxilan is a contrast agent and small molecule drug developed by Guerbet SA. It is primarily used for the treatment of vascular diseases in the field of biomedicine. With its approval status and first approval in Japan in 1995, Ioxilan has established itself as a valuable tool in diagnosing and monitoring cardiovascular conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
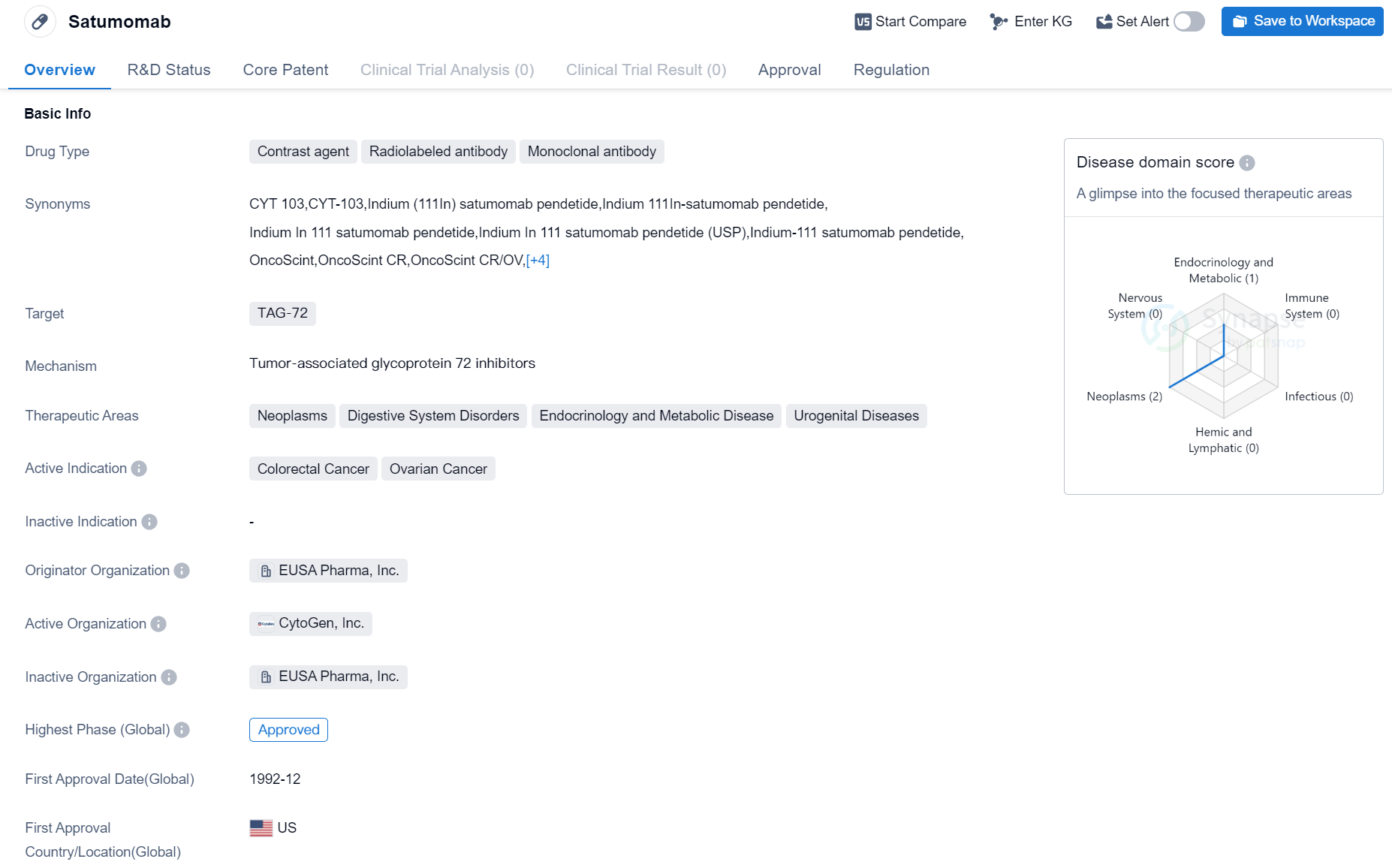
Approved Contrast Agent Medicinal: Satumomab
Satumomab is a drug classified as a contrast agent, radiolabeled antibody, and monoclonal antibody. It is primarily used in the field of biomedicine and is designed to target TAG-72, a protein found in certain types of cancer cells. The drug has been approved for use in the treatment of neoplasms, digestive system disorders, endocrinology and metabolic diseases, and urogenital diseases.
Satumomab is specifically indicated for the treatment of colorectal cancer and ovarian cancer. It was first approved for use in December 1992 in the United States. The drug is developed and marketed by EUSA Pharma, Inc., an originator organization in the pharmaceutical industry.
In terms of regulatory status, Satumomab is classified as an orphan drug. Orphan drugs are medications that are developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives and benefits to the manufacturer, such as extended market exclusivity and financial support for research and development.
The highest phase of development for Satumomab is approved, indicating that it has successfully completed clinical trials and has been granted regulatory approval for commercialization. This suggests that the drug has demonstrated safety and efficacy in treating the approved indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, Satumomab is a contrast agent, radiolabeled antibody, and monoclonal antibody drug that targets TAG-72. It is approved for the treatment of colorectal cancer and ovarian cancer. Developed by EUSA Pharma, Inc., the drug received its first approval in the United States in 1992 and is regulated as an orphan drug. With its highest phase being approved, Satumomab has shown promising results in clinical trials and is available for use in the treatment of specific cancers.