T-cell co-stimulation target: 4-1BB (CD137)
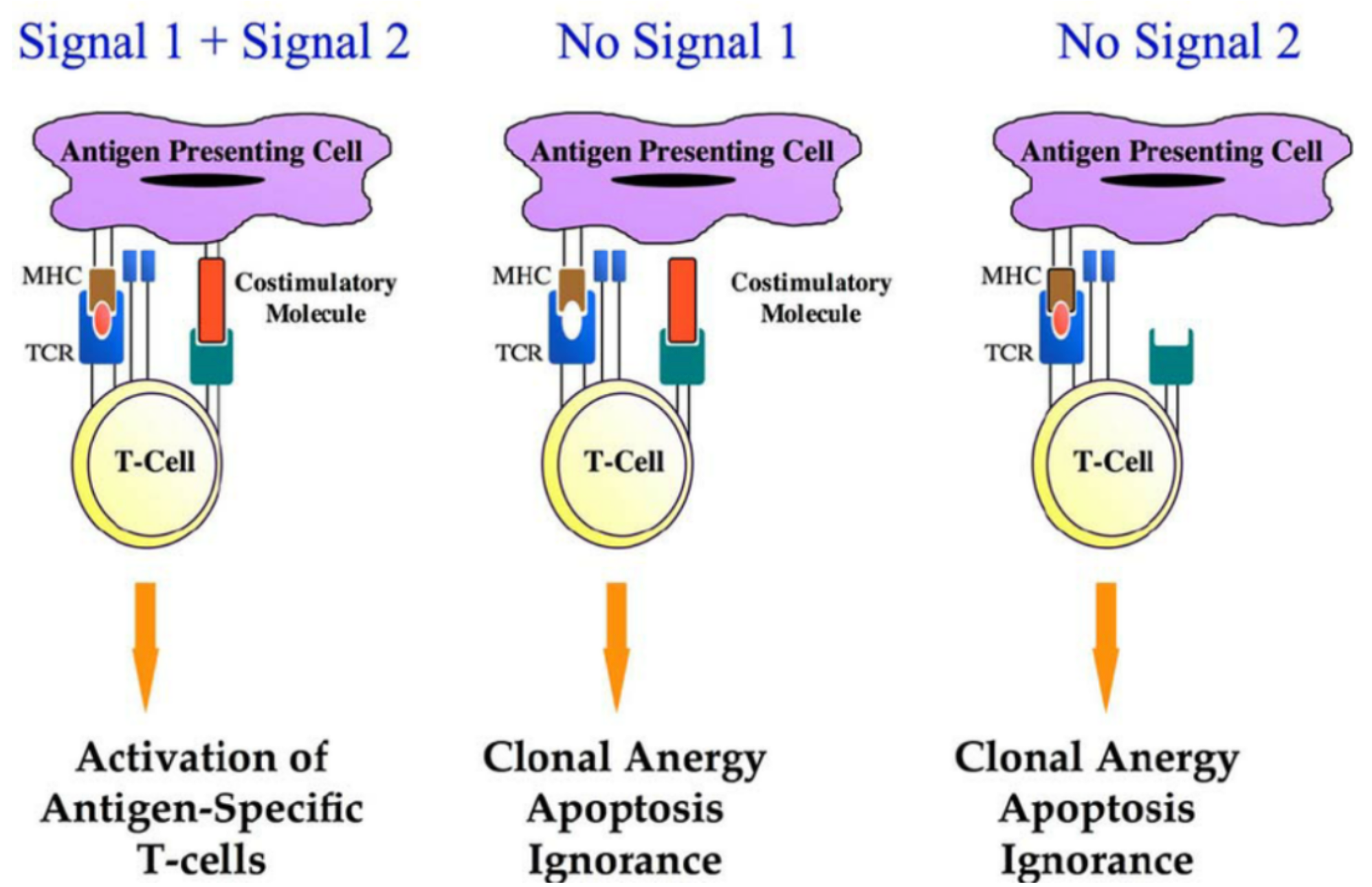
T-cell immune responses play a vital role in the control of tumors. To induce robust and durable T-cell immune responses, the initial requirement is a signal induced by T-cell receptor (TCR) activation (Signal 1). Signal 1 is antigen-specific and it is achieved via the interaction between the T-cell receptor and the peptide-major histocompatibility complex (MHC) on the antigen-presenting cell (APC).
However, Signal 1 alone is not sufficient to effectively activate T-cell immune responses, and a sufficient co-stimulatory signal (Signal 2) is required. Unlike Signal 1, Signal 2 is not antigen-specific. Signal 2 is achieved through the interaction between the co-stimulatory signal molecules expressed on APCs and T cells. Costimulatory signals are necessary for T-cell proliferation, differentiation, and survival. Without costimulatory signals, there may be an unresponsive/low response T cells, cell death, or the emergence of immune tolerance.
Common co-stimulatory molecule protein families include the IgSF (Immunoglobulin Superfamily), TNRSF (Tumor Necrosis Factor Receptor Superfamily), in addition to others derived from the TIM family, CD2/SLAM family of costimulatory factors. Due to the critical role of costimulatory factors in T-cell immune activation, they have been of particular interest in the field of tumor therapy.
4-1BB
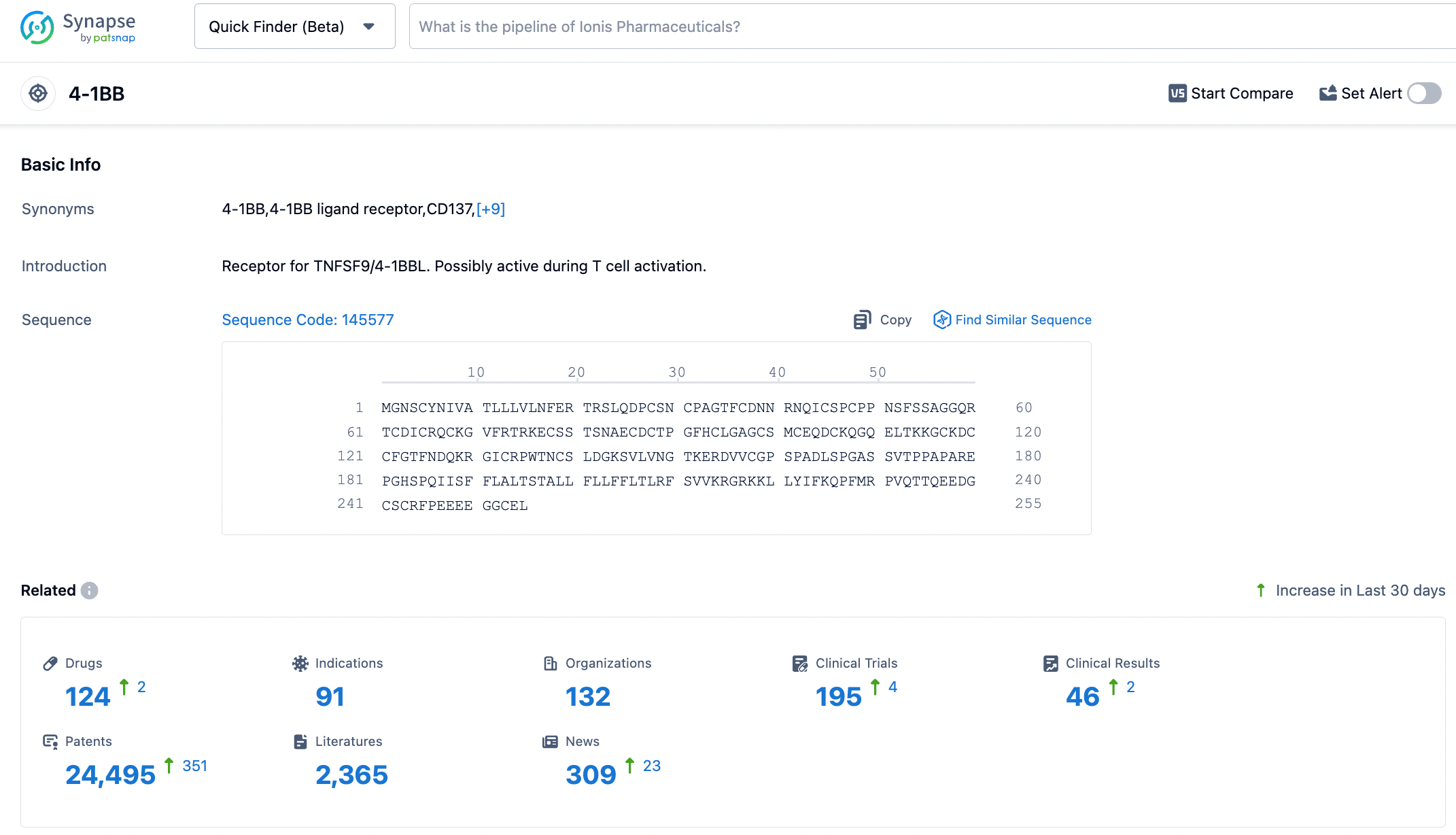
4-1BB (also known as CD137 or TNFRSF9) was first discovered in 1989 and is a critical co-stimulation receptor on T cells and other immune cells. It is a member of the TNRSF protein family mentioned earlier.
In 1997, Melero and colleagues found that a monoclonal antibody against 4-1BB was able to induce anti-tumour activity in T cells in mice. The main effects of 4-1BB on T cell activation include cell proliferation, cytokine release, cytotoxicity, and the formation of long-term immune memory, among others. Most of T cell activation occurs in CD8+ T cells.
Two second-generation CAR-T cell therapies, Kymriah (Novartis) and CARVYKTI (Johnson&Johnson), which have been approved by the FDA, utilise 4-1BB as their co-stimulation domain. Other second-generation CAR-T therapies use CD28 as their co-stimulatory domain. Although both have shown similar tumour control rates in cancer patients, some differences were found: CD28 appears to have a higher cytokine release effect and can better promote T cell proliferation, but it also has a higher risk of neurotoxicity, while 4-1BB can foster longer-term T cell persistence.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
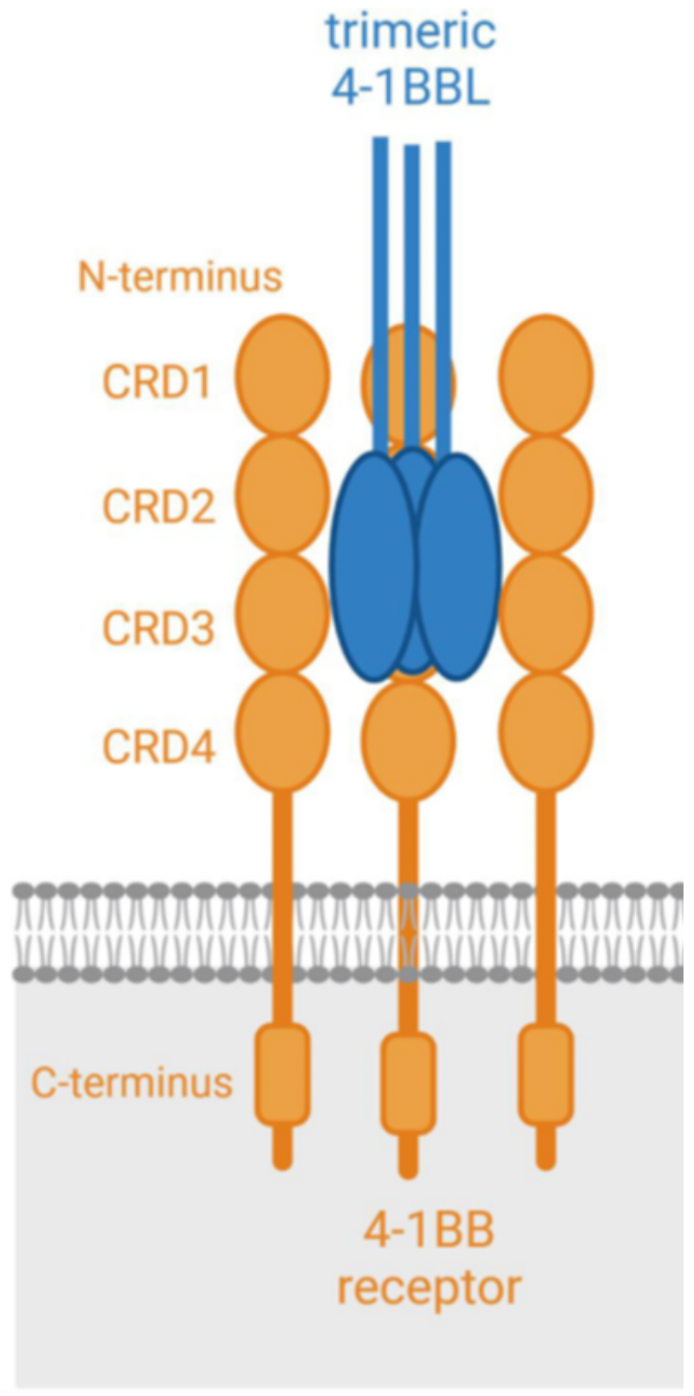
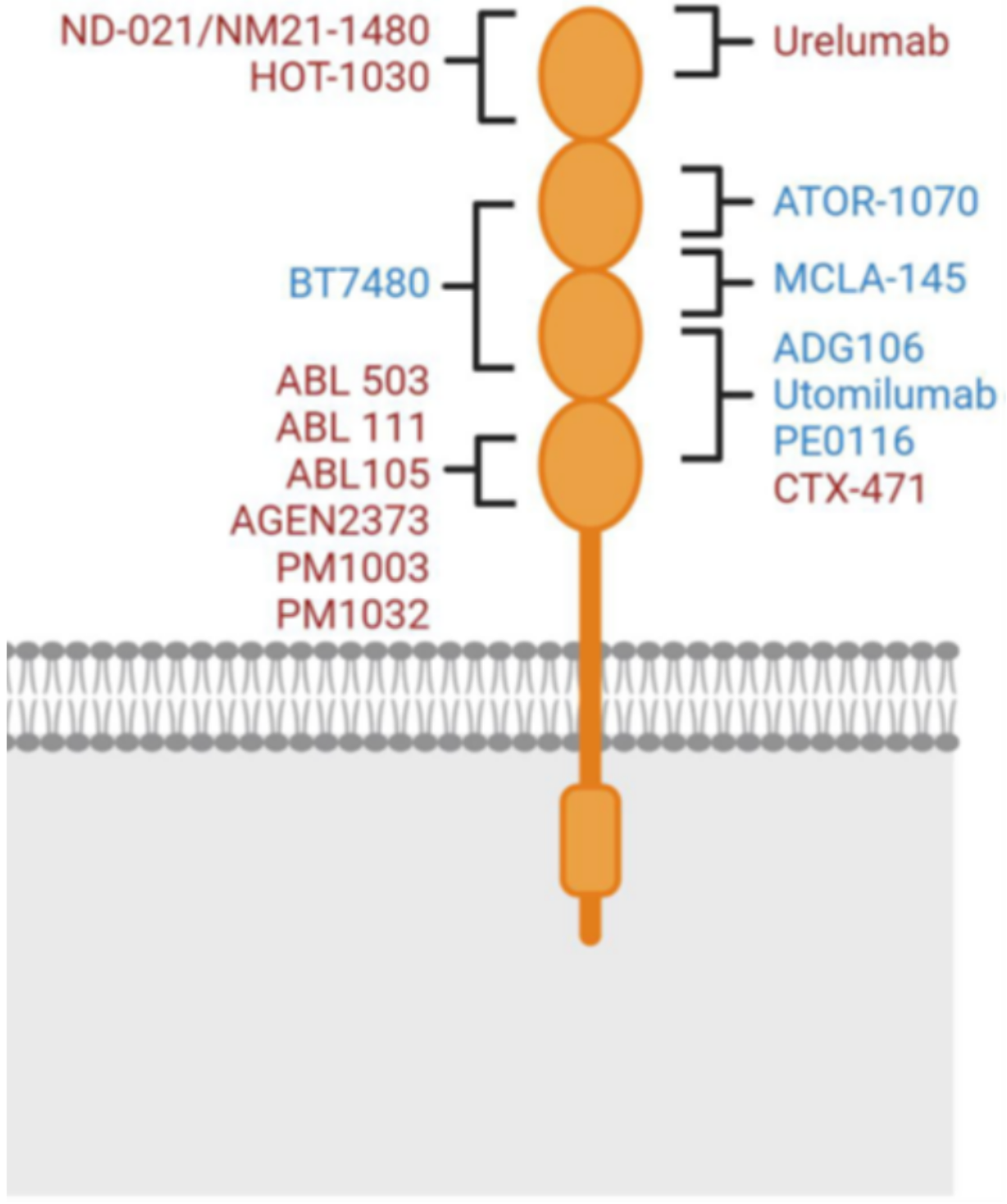
4-1BB is a glycosylated type-I membrane protein. It has four functional domains (known as "CRD", cysteine-rich pseudo repeats domain). The 4-1BB ligand (4-1BBL) is the binding partner of 4-1BB, which is generally expressed on the cell surface as a homotrimeric complex. Upon antigen stimulation, effective binding between 4-1BB and 4-1BBL can activate rapid receptor activation. For optimal co-stimulation (signal 2), 4-1BB needs to cluster on the cell surface.
The Application of 4-1BB in Antibody Drug Development
The First Generation of 4-1BB Agonist Drugs
The clinical development of 4-1BB agonist antibodies began in 2005 with the antibody drug urelumab (BMS-663513) created by Bristol-Myers Squibb (BMS). Urelumab is a humanized anti-4-1BB IgG4 subtype antibody. The early development data was promising, but there were two serious adverse events due to hepatic toxicity. Subsequent research found that while the drug was effective, its potency was very limited when administered at a safe dose (0.1 mg/kg).
The second 4-1BB antibody drug developed was Pfizer's utomilumab (PF-05082566). The antibody drug is a fully human IgG2 subtype antibody and entered clinical trials in 2011. Although it did not demonstrate the safety issues seen in urelumab, its effectiveness (whether used alone or in combination with rituximab) was very limited, leading to the discontinuation of its clinical development.
Urelumab and utomilumab, as the first generation of 4-1BB antibodies, displayed different agonistic activities and liver toxicities. These differences are currently thought to be due to differences in the Fc effector functions and epitope binding of these two molecules. The Fc effector function, particularly binding to FcγRIIB, is generally believed to be associated with liver toxicity (FcγRIIB cross-linking in the liver). As an IgG4 subtype, urelumab has stronger FcγRIIB binding, which is likely why it differs in liver toxicity from utomilumab (IgG2 subtype). The epitope binding is another reason for the different activities between urelumab and utomilumab. Urelumab and 4-1BB bind through an epitope on CRD1, which does not interfere with the binding of 4-1BB to its natural ligand.
Utomilumab binds to 4-1BB through epitopes on CRD2 and CRD3. This binding method results in a large amount of endogenous 4-1BB ligand competing with utomilumab for binding to the 4-1BB receptor, limiting the activity of 4-1BB receptor clustering (which promotes the agonist activity of 4-1BB). This explains why urelumab (which does not affect 4-1BB receptor clustering) has higher agonist activity than utomilumab (which does affect 4-1BB receptor clustering).
Second Generation 4-1BB Agonist Drugs
The mediocre performance of Urelumab and Utomilumab in clinical trials did not douse the interest of many pharmaceutical companies in further exploring the 4-1BB target. Since the beginning of 2017 to the present, more than 40 4-1BB agonist drugs have entered clinical trials. Although the clinical trials of 4-1BB drugs temporarily declined in 2020 due to the COVID-19 pandemic, they showed a strong rebound afterwards. There may be more and more 4-1BB agonist drugs entering the clinic in the future, as many pharmaceutical/biotech companies are actively conducting preclinical research on 4-1BB drugs.
The 4-1BB drugs that appeared after Urelumab and Utomilumab are categorized as the second-generation 4-1BB agonist drugs in this article. The second-generation 4-1BB agonist drugs mainly fall into two categories: IgG type (mono-antibody structure) and bi/multi-antibody 4-1BB agonist drugs.
The mechanism of action of the IgG type second-generation 4-1BB agonist drugs has some consistency with the first-generation drugs (Urelumab and Utomilumab): they mostly rely on the cross-linking effect of Fcγ receptors, but their binding epitopes have been modified compared to the first generation. For example, the drug ADG106 developed by Suzhou Tyvance Biotech, also adopts the IgG4 subtype like Urelumab, but it does not bind to Urelumab's epitope CRD1. The concept behind this design is to have the drug be effective while reducing its toxic side effects, achieving a more precise regulation of the balance between effectiveness and safety (“sweet point” of functionality).
The second type of second-generation 4-1BB agonist drugs are bi/multi-antibody agonist drugs. These drugs bind to at least one other target in addition to 4-1BB. Adding another target can increase the specificity of the drug, its cross-linking effect, and confer additional stimulatory or inhibitory activity, endowing the drug with extra anti-tumor properties. Targets commonly paired with 4-1BB include: PD-L1 (e.g., I-MAB Biopharma's TJ-L14B, Nanjing Leads Biolabs's LBL-024, Biotheus' PM1003), FAP (e.g., Roche's RG7827, Boehringer Ingelheim's BI765179) etc. In addition, the Fc end of this type of drug has been engineered to eliminate the ability to bind with Fcγ receptors but maintain the ability to bind with neonatal Fc receptors (FcRn) to ensure the half-life of the drug in the body. The aim is to suppress the liver toxicity seen in Urelumab, improving the safety of the drug. Drugs adopting this design strategy include Roche's RG7827 and RG6076, Elpiscience Biopharma's ES101, I-MAB Biopharma's TJ-L14B etc.
Immunotherapy, surgery, radiation therapy, and chemotherapy together constitute the four methods of cancer treatment. In order to enhance the efficacy, surgery/chemotherapy/radiation therapy can be combined with immunotherapy as a joint treatment strategy. Currently, the combination of 4-1BB with radiation therapy and chemotherapy has been shown to have a good synergistic effect. In addition, anti-4-1BB antibodies have also been shown to improve the efficacy of other antibodies. Joint treatment plans based on 4-1BB antibodies may be a direction for future advancements in cancer treatment.