Zanidatamab Receives Priority Review for HER2+ Advanced Biliary Tract Cancer
Jazz Pharmaceuticals plc revealed that the U.S. Food and Drug Administration (FDA) has accepted and given Priority Review status to the Biologics License Application (BLA) for zanidatamab. This HER2-targeted bispecific antibody is intended for treating patients with previously treated, unresectable, locally advanced, or metastatic HER2-positive biliary tract cancer. As per the Prescription Drug User Fee Act, the FDA has set a goal action date of November 29, 2024.
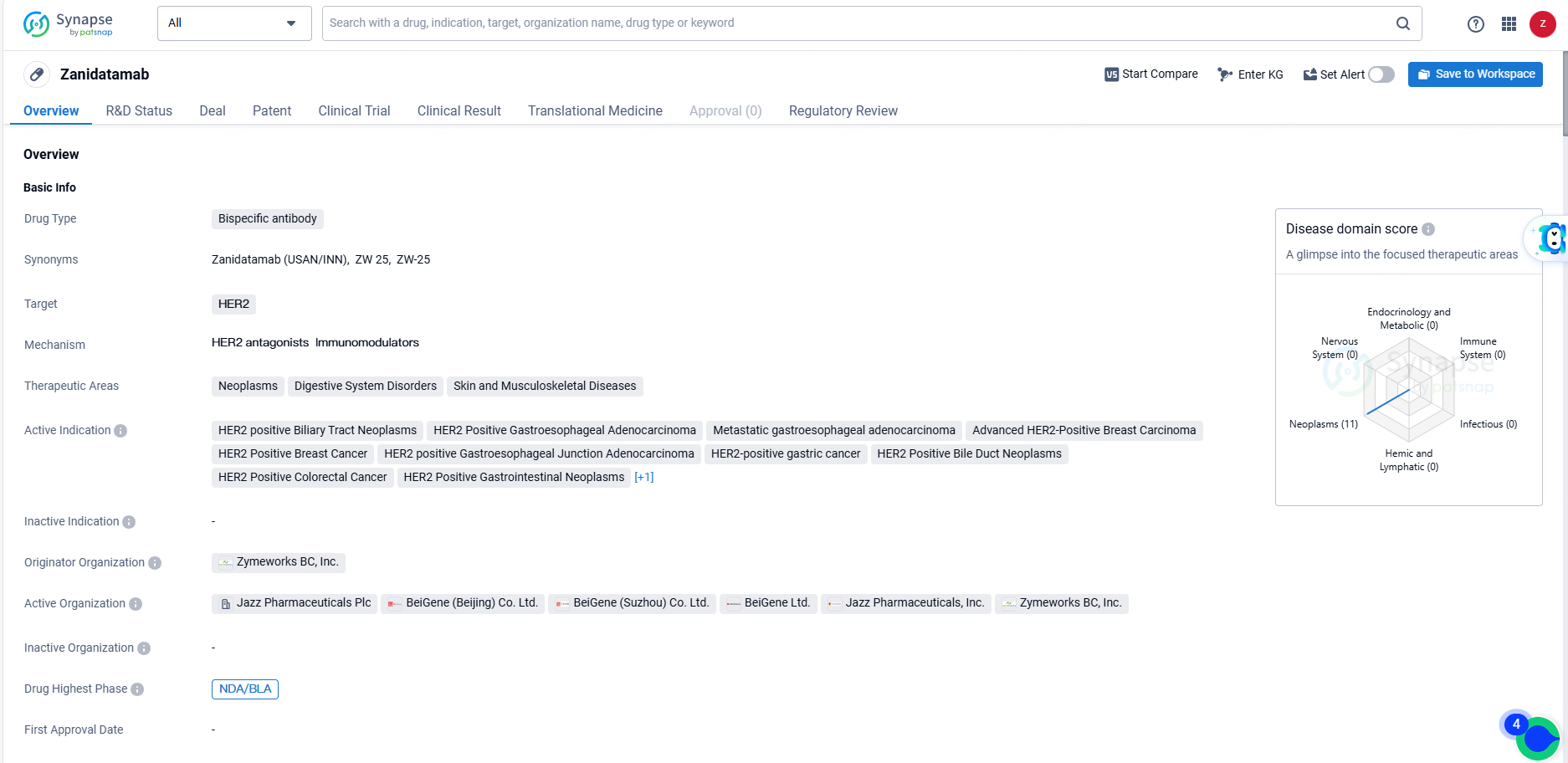
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The prioritization of zanidatamab for review highlights the urgent need for novel treatments for patients with locally advanced or metastatic HER2-positive BTC, a severe illness with a grim outlook," stated Rob Iannone, M.D., M.S.C.E., Executive Vice President and Global Head of Research and Development at Jazz Pharmaceuticals. "Should it receive approval, zanidatamab will be the inaugural HER2-targeted therapy specifically authorized for these patients, and we are eager to provide this new treatment option to the BTC community."
Jazz's Biologics License Application (BLA) is supported by data from Cohort 1 of the Phase 2b HERIZON-BTC-01 clinical trial, which evaluated zanidatamab in patients with unresectable, locally advanced, or metastatic HER2-positive BTC who had previously undergone treatment. The trial met its primary endpoint with a 41.3% confirmed objective response rate as assessed by independent central review. These findings were showcased at the American Society of Clinical Oncology (ASCO) Annual Meeting 2023, published in The Lancet Oncology, and featured in the 2023 Best of ASCO program.
Additional long-term data, including overall survival and updated duration of response from the Phase 2b HERIZON-BTC-01 trial, will be disclosed at the forthcoming ASCO Annual Meeting 2024.
Furthermore, the global, open-label, randomized Phase 3 HERIZON-BTC-302 trial, which is evaluating the safety and effectiveness of zanidatamab in conjunction with standard-of-care therapy compared to standard-of-care therapy alone for first-line advanced or metastatic HER2-positive BTC, is currently underway and accepting participants. HERIZON-BTC-302 is intended to serve as the pivotal confirmatory study for zanidatamab in BTC.
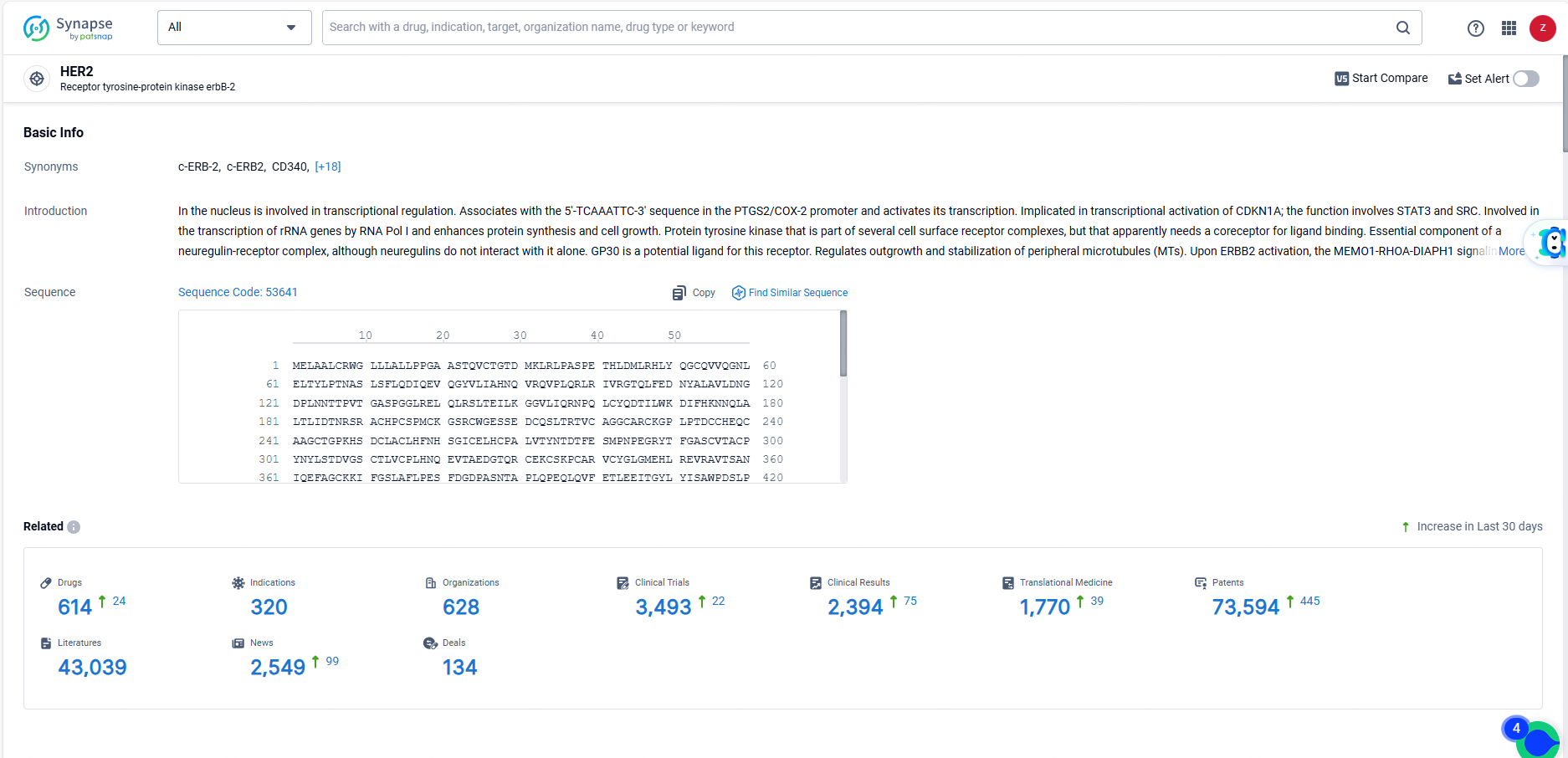
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 3, 2024, there are 614 investigational drugs for the HER2 target, including 320 indications, 628 R&D institutions involved, with related clinical trials reaching 3493, and as many as 73594 patents.
Zanidatamab shows promise as a potential treatment for a variety of HER2-positive cancers, and its regulatory designations indicate that it may offer significant benefits to patients in need. As it progresses through the development and approval process, Zanidatamab has the potential to make a meaningful impact in the field of biomedicine and improve outcomes for patients with HER2-positive cancers.