European Commission Approves FILSPARI® by CSL Vifor and Travere Therapeutics for IgA Nephropathy Treatment
CSL Vifor along with Travere Therapeutics, Inc., have disclosed that the European Commission approved a conditional marketing authorization for FILSPARI (sparsentan), targeted at treating primary IgAN in adults exhibiting a urinary protein excretion of 1.0 g/day or more. This authorization encompasses all member countries within the European Union, in addition to Iceland, Liechtenstein, and Norway.
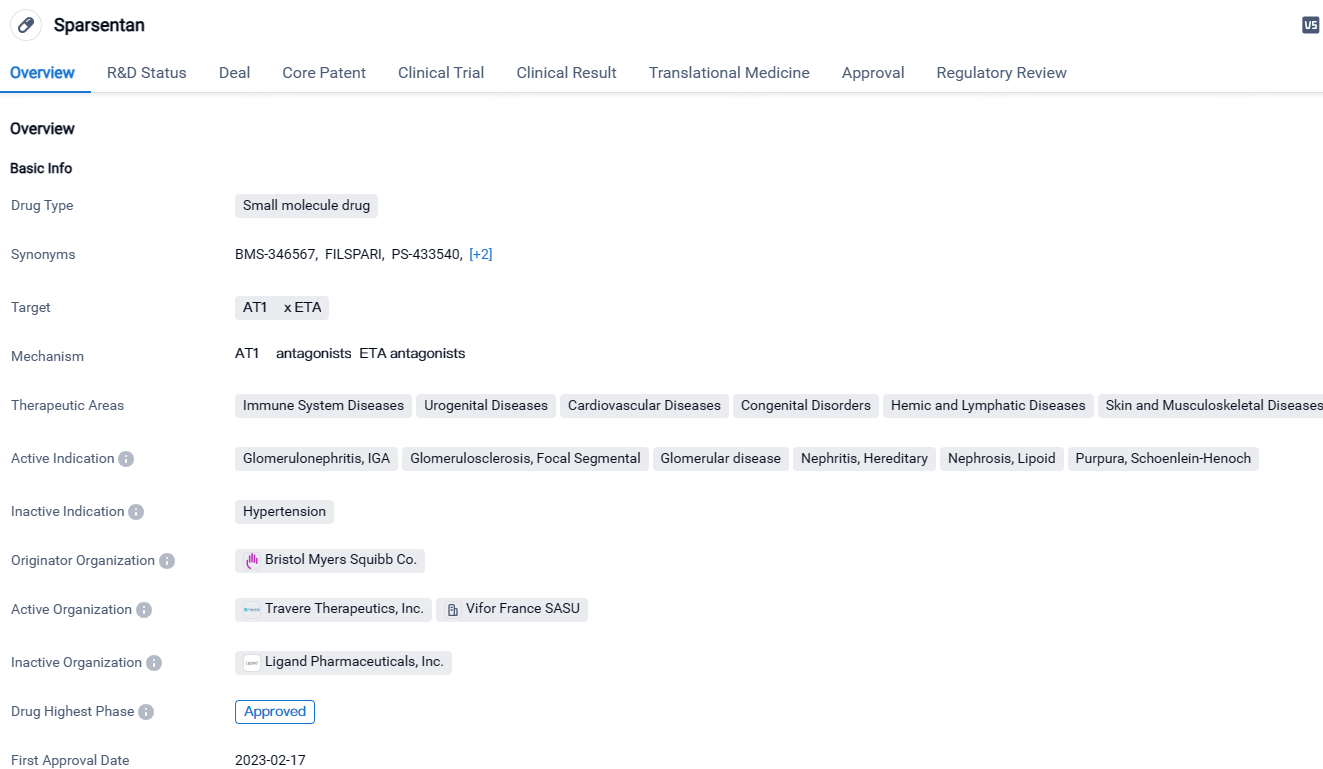
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"In a significant advancement for European patients grappling with IgAN—a severe and infrequent disease that is a predominant cause of end-stage renal disease—Prof. Dr. med. Jürgen Floege, a Senior Professor of Nephrology and Clinical Immunology at the University Hospital, RWTH Aachen, Germany, and a member of the steering committee for the PROTECT clinical trial, stated: 'The introduction of this pioneering treatment, rooted in findings from the first-ever phase-III head-to-head clinical trial in IgAN, opens new doors for adult patients at severe risk of advancing to kidney failure by offering an effective therapy that greatly diminishes proteinuria and decelerates renal disease progression," RWTH Aachen reported.
"Securing approval from the European Commission marks a crucial turning point for the IgAN community in Europe and illustrates our commitment to pioneering and furnishing cutting-edge treatments in areas of great unmet need," commented Emmanuelle Lecomte Brisset, Senior Vice President and Head of Global Regulatory Affairs at CSL. "We are eager to collaborate with our partners and the EU member states to make this innovative therapy available to European patients."
"Emphasizing the distinction of FILSPARI as the first and sole non-immunosuppressive therapy greenlit for IgAN, Eric Dube, Ph.D., President and CEO of Travere Therapeutics, remarked, 'FILSPARI is set to become a new cornerstone treatment for this rare renal disorder, potentially taking over from RAAS inhibition strategies. With CSL Vifor's robust commercial capabilities, we anticipate enabling patients across Europe afflicted with IgAN to benefit from this crucial therapy.'"
The favorable stance from the European Commission comes after the CHMP endorsed FILSPARI in February 2024, following compelling results from the milestone phase-III PROTECT study of FILSPARI in IgAN. The study achieved its primary endpoint with statistical significance at a predetermined interim analysis. After 36 weeks, patients treated with FILSPARI experienced a significant average proteinuria reduction of 49.8 percent from baseline, contrasted with a 15.1 percent reduction in those treated with irbesartan.
Further, the two-year confirmatory outcomes demonstrated that FILSPARI treatment significantly outperformed irbesartan on the chronic eGFR slope endpoint and showed meaningful preservation of kidney function.
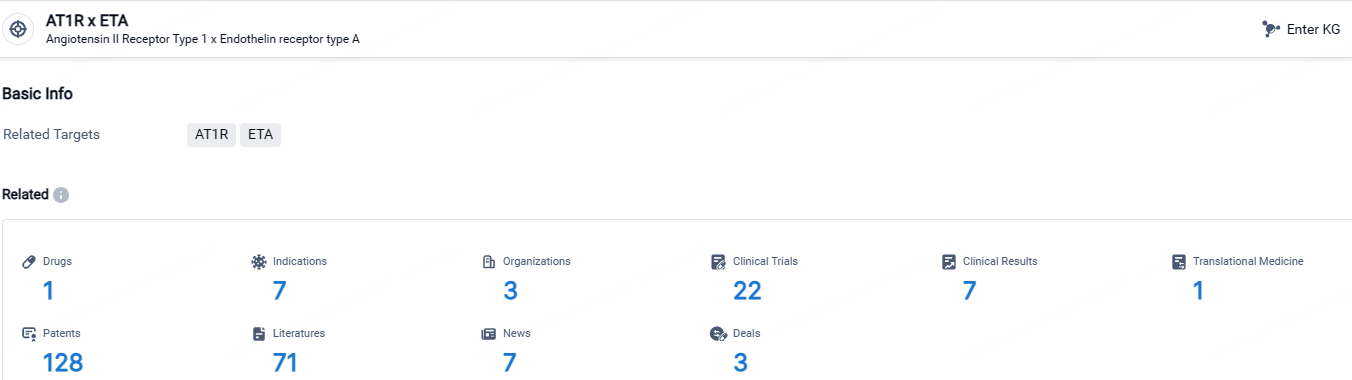
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 26, 2024, there are 1 investigational drugs for the AT1R and ETA targets, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 22, and as many as 128 patents.
Sparsentan is a small molecule drug that targets AT1R x ETA receptors. It has received approval for the treatment of various conditions related to immune system diseases, urogenital diseases, cardiovascular diseases, congenital disorders, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The drug has achieved its first approval in the United States and has undergone priority review, accelerated approval, and orphan drug designation. Sparsentan represents a significant advancement in the field of biomedicine, particularly in addressing kidney and immune system disorders.