European Marketing Approval Granted to Pierre Fabre's OBGEMSA™ for Overactive Bladder Treatment
The European Commission has given approval to Pierre Fabre Laboratories to market OBGEMSA™ (vibegron) for managing symptoms of overactive bladder syndrome in adults, a condition that affects over 70 million people* throughout Europe and is notably distressing.
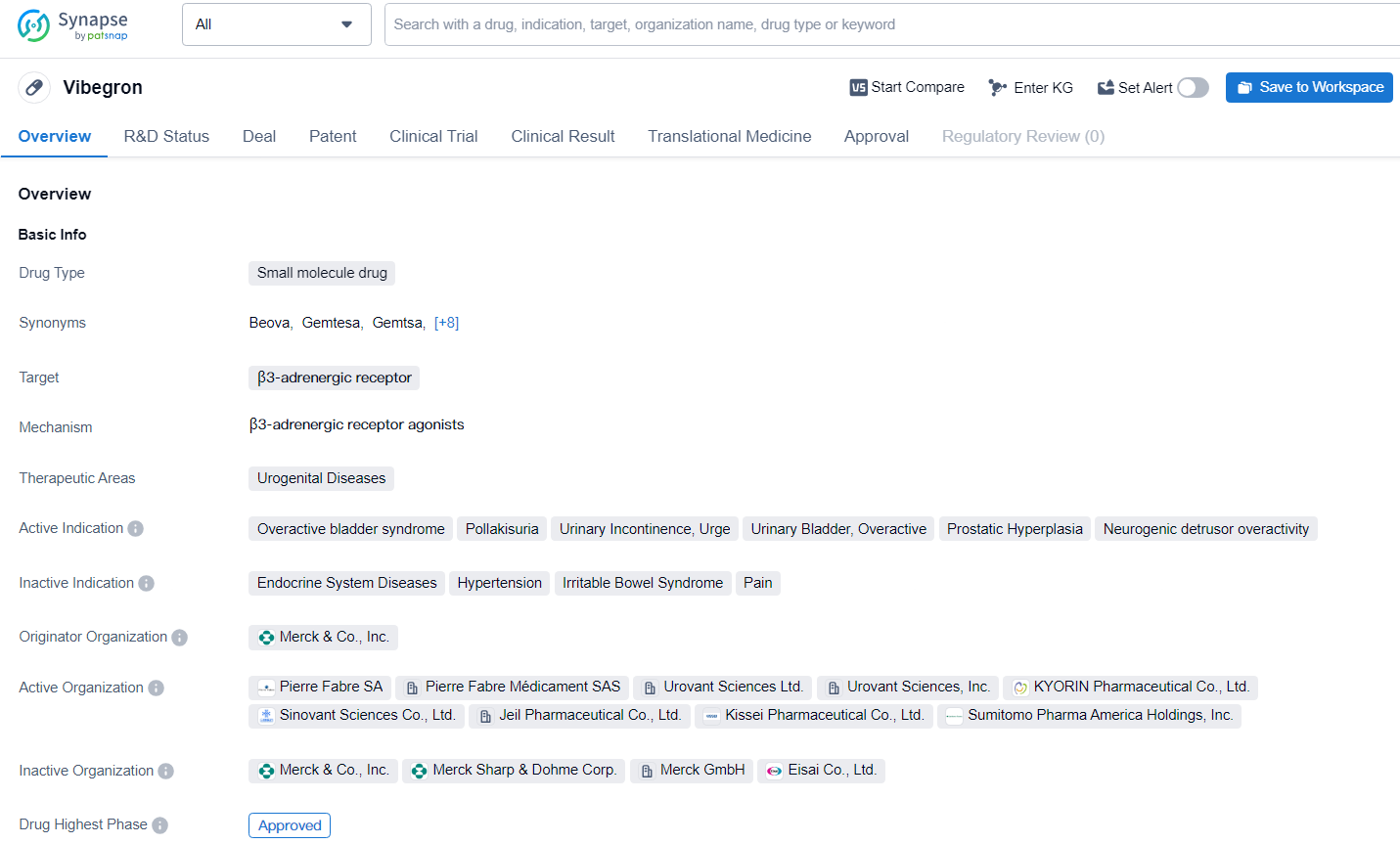
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In 2022, Pierre Fabre Laboratories obtained the exclusive rights from Urovant Sciences Gmbh to register and commercialize vibegron within the European Economic Area. This decision by the European Commission (EC) will apply to all EU member countries, as well as Iceland, Liechtenstein, and Norway. The trademark OBGEMSA™ is owned by Urovant Sciences.
“We are thrilled with this progress, which will enable European patients to access a new treatment option for overactive bladder syndrome. This will further enhance our over 40 years of expertise in urology. The decision underscores Pierre Fabre Laboratories’ dedication to providing patients with innovative therapies that improve the management of chronic debilitating conditions,” stated Eric Ducournau, CEO of Pierre Fabre Laboratories.
The EC's decision is based on a favorable opinion issued by the Committee for Medicinal Products for Human Use of the European Medicines Agency on April 25. It relies on the findings from two critical, multicenter, double-blind, randomized phase 3 trials involving adults with overactive bladder symptoms. Study RVT-901-3003 assessed the 12-week efficacy, tolerability, and safety of vibegron compared to placebo and tolterodine as a positive control. Its extension, study RVT-901-3004, examined vibegron's long-term safety, tolerability, and efficacy over 52 weeks in a double-blind manner, using tolterodine as an active comparator.
In these studies, vibegron, a novel selective beta-3 adrenergic receptors (AR) agonist, showed a favorable benefit-risk profile for treating symptoms such as urgency, increased urination frequency, and urge urinary incontinence in patients with overactive bladder syndrome.
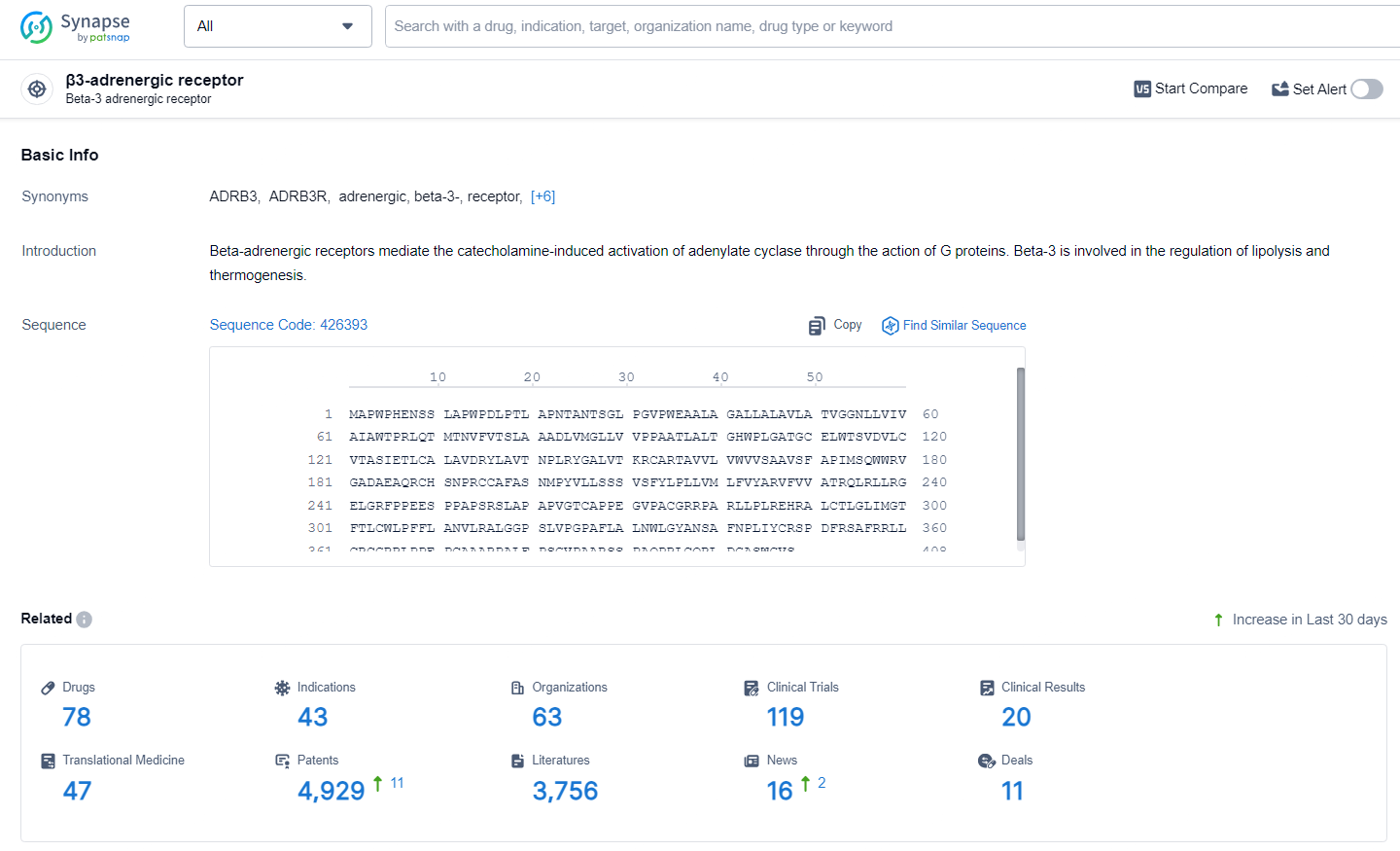
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of Julye 3, 2024, there are 78 investigational drugs for the β3 adrenergic receptors, including 43 indications, 63 R&D institutions involved, with related clinical trials reaching 119, and as many as 4929 patents.
Vibegron represents a significant advancement in the pharmaceutical industry's efforts to address urogenital diseases, particularly in the treatment of overactive bladder syndrome and related indications. Its approval and ongoing development underscore the importance of continued research and innovation in the field of biomedicine to improve patient outcomes and quality of life.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!