Evaxion secures initial pre-clinical validation for its mRNA Gonorrhea vaccine candidate, EVX-B2
Evaxion Biotech A/S (NASDAQ: EVAX) (“Evaxion”), a technology-driven biotechnology firm in the clinical stage that focuses on creating AI-Immunology™ based vaccines, has released new pre-clinical results. These findings showcase the efficacy of its innovative EVX-B2 mRNA Gonorrhea vaccine candidate in eradicating gonorrhea bacteria through the induction of a specialized immune response.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Today at the 18th Vaccine Congress in Lisbon, Portugal, new data will be unveiled demonstrating strong Proof-of-Concept (PoC) for the mRNA-based iteration of EVX-B2 in a pre-clinical environment.
Originally, EVX-B2 was engineered as a protein-based vaccine candidate, which had already achieved pre-clinical PoC. The latest pre-clinical findings for the mRNA-based version of the vaccine confirm that AI-Immunology™ identified antigens are versatile across different vaccine technologies. The data were generated in partnership with Afrigen Biologics.
“We are excited to announce the pre-clinical PoC for the mRNA version of EVX-B2, which marks another milestone for our 2024 objectives. It is gratifying to witness further validation of our AI-Immunology™ platform and its potential in developing innovative treatments, including for bacterial diseases. Currently, there is no existing vaccine for Gonorrhea, so this could lead to a pioneering solution. Additionally, this data reaffirms that our platform is versatile in terms of delivery modalities, presenting a compelling value proposition for both current and prospective partners,” stated Christian Kanstrup, CEO of Evaxion.
Petro Terblanche, CEO of Afrigen Biologics, also expressed enthusiasm about the outcomes of the collaboration: “With rising global challenges in antimicrobial resistance and the public health priority of effective interventions against increases in Gonorrhea cases, we are thrilled to have reached pre-clinical PoC for EVX-B2’s mRNA version, which shows promise as a future vaccine product.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
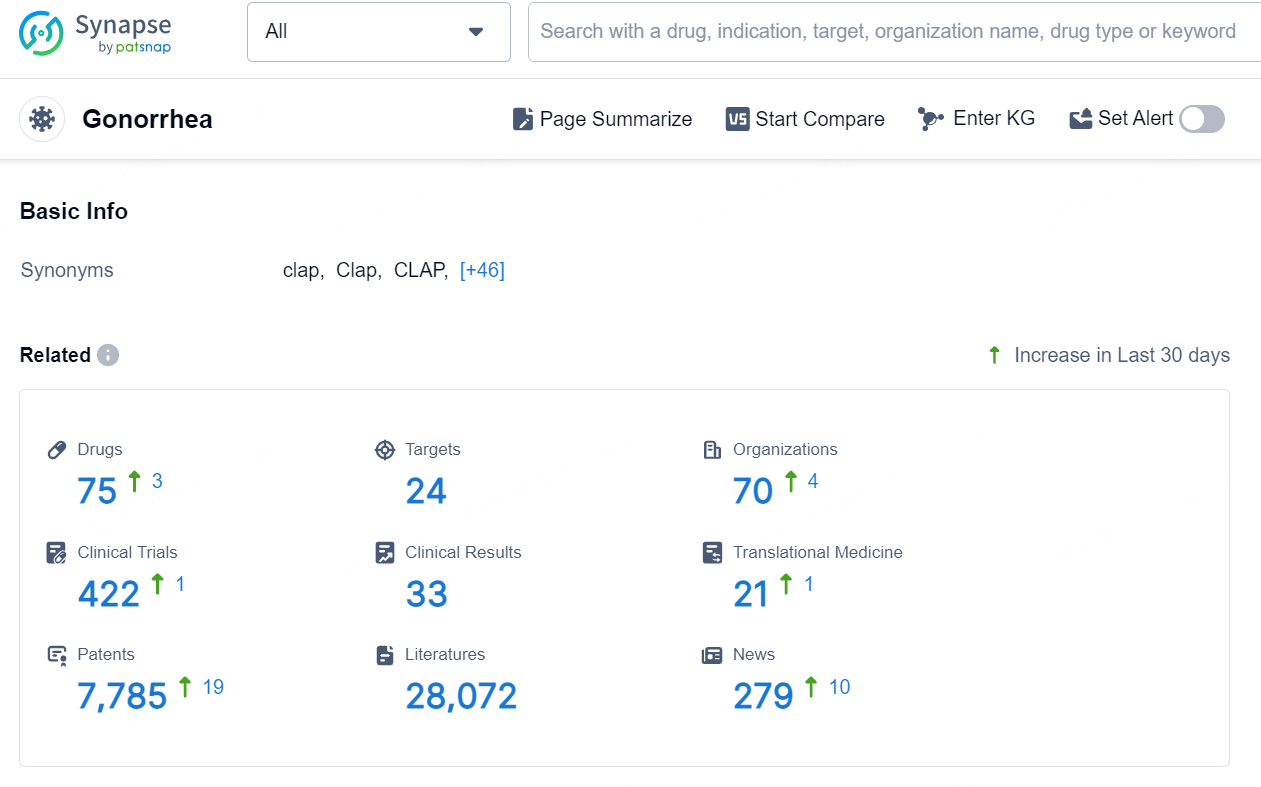
According to the data provided by the Synapse Database, As of September 11, 2024, there are 75 investigational drugs for the Gonorrhe, including 24 targets, 70 R&D institutions involved, with related clinical trials reaching 422, and as many as 7785 patents.
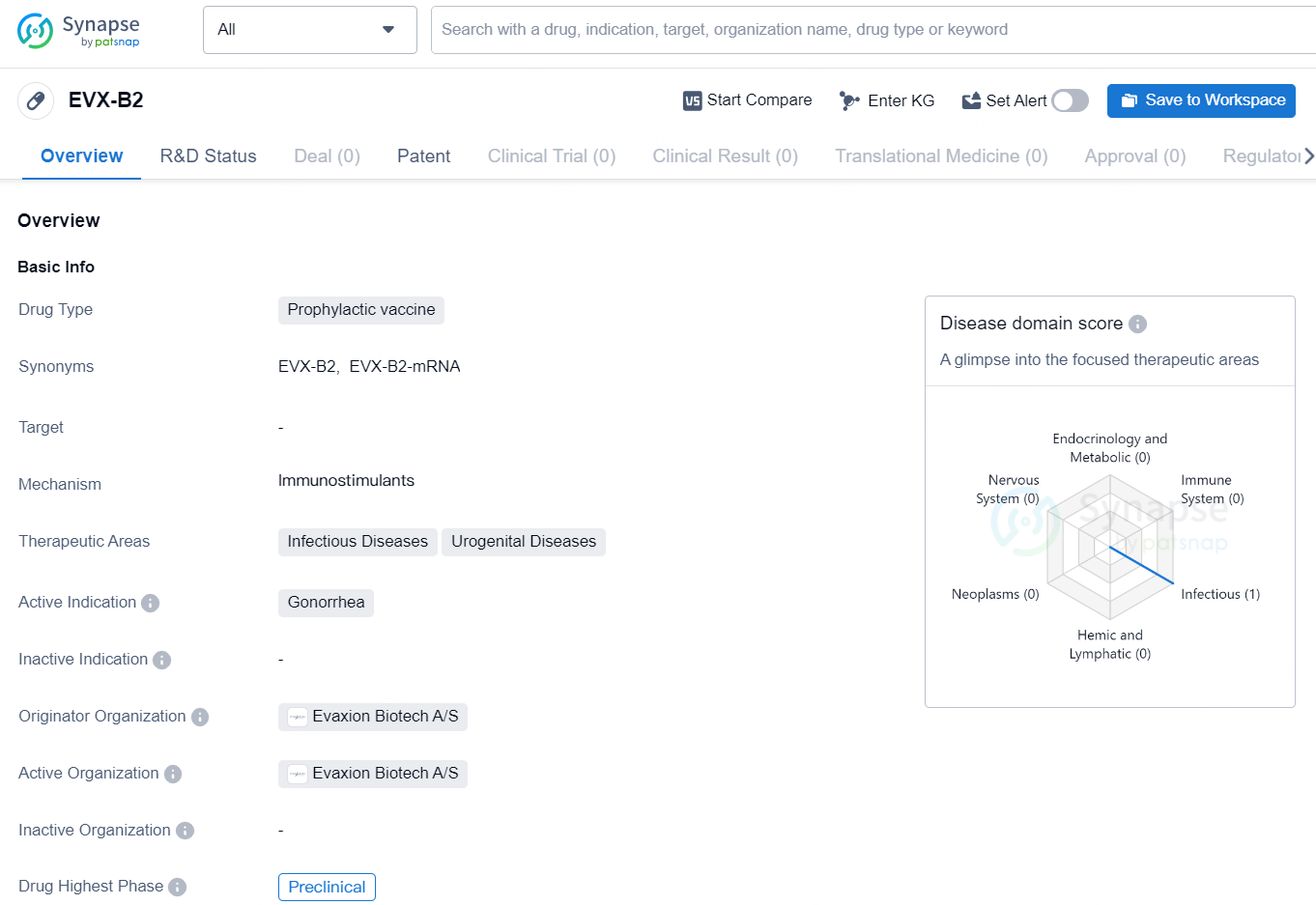
EVX-B2 is a prophylactic vaccine being developed by Evaxion Biotech A/S for the prevention of gonorrhea, which falls under the therapeutic areas of Infectious Diseases and Urogenital Diseases. The drug is currently in the preclinical phase, meaning it is undergoing laboratory and animal testing to determine its safety and efficacy before advancing to clinical trials in humans.