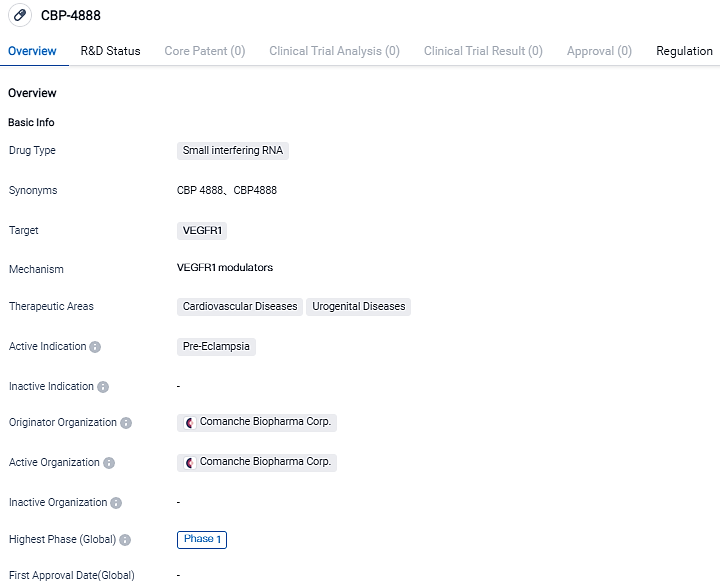
FDA Fast Track Status Granted to Comanche Biopharma's CBP-4888 for Early-Onset Preeclampsia Treatment
Comanche Biopharma Corp. has announced today they obtained Fast Track status from the U.S. Food and Drug Administration for the assessment of CBP-4888, a treatment candidate for sFlt1-induced early-onset preeclampsia.CBP-4888 is an siRNA therapeutic, administered subcutaneously and presently part of a Phase 1 clinical trial.
The Fast Track scheme by FDA aims to enhance the development process and speed up the evaluation of original potential treatments meant to address severe illnesses which are currently without adequate medical solutions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Comanche Biopharma appreciates the FDA's recognition of the potential our novel siRNA investigational medication, CBP-4888, holds to mitigate the rapidly rising maternal health disaster," expressed Scott Johnson, M.D. He is the Co-Founder and CEO of the company. "This endorsement fosters expanded interaction with the FDA to facilitate the speedy delivery of CBP-4888 to patients whilst adhering to the strictest safety and quality norms."
Preeclampsia impacts over 10 million women globally each year, having terrible immediate and long-term repercussions on both the mother and baby. At this time, no current therapy can influence the progression of preeclampsia. Allison August, M.D., Chief Medical Officer of Comanche Biopharma, states, “Preeclampsia complications account for about 80,000 maternal and 500,000 fetal and newborn fatalities worldwide each year.
At present, the sole remedy for preeclampsia is early delivery. The FDA Fast Track designation awarded today moves us a step further towards our mutual objective of promoting a secure and effective treatment alternative for preeclampsia sufferers.”
Comanche's dedication lies in reducing pregnancy and prematurity risks globally through maintaining natural pregnancy safely.
About CBP-4888
CBP-4888 comprises a fixed dosage of a pair of chemically created, lipid-conjugated small interfering ribonucleic acid (siRNAs) duplex oligonucleotides (siRNA-2283 and siRNA-2519) that target two soluble fms-like tyrosine kinase–1 (sFLT1) mRNA isoforms.
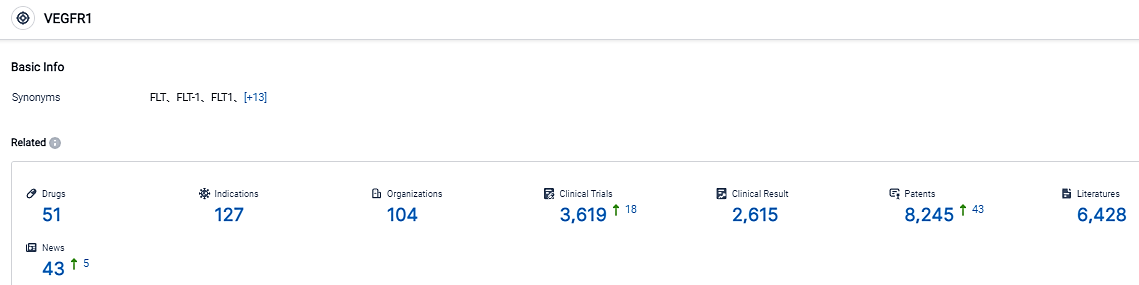
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 4, 2023, there are 51 investigational drugs for the VEGFR1 target, including 127 applicable indications, 104 R&D institutions involved, with related clinical trials reaching 3619, and as many as 8245 patents. Preeclampsia is a critical and widespread obstetrical issue that impacts roughly 8% of pregnancies at a global level. This condition can cause a series of adverse effects for both the maternal and fetal well-being such as multi-organ trauma, convulsions, and preterm labor.
Each year across the world, preeclampsia is believed to cause about 80,000 maternal deaths and 500,000 late-term miscarriages, fetal or neonatal deaths. Although it can occur in any pregnancy, preeclampsia is disproportionately observed in African descent, populations in low-to-middle income nations, and individuals living in rural communities.
Characteristics of preeclampsia are not rigid and might comprise elevated blood pressure, acute kidney damage, body extremities and facial edema, intense headaches, visual disturbances, and belly discomfort.
Therapeutic strategies could encompass stringent control of blood pressure and urinary protein concentrations, bed confinement, and drugs to reduce blood pressure. To this date, the only certain resolution for preeclampsia is childbirth.