Exosomes and Tumor Immunotherapy
Research on tumor immune evasion and tolerance mediated by exosomes has found that tumor cells can produce a large amount of exosomes. Exosome-mediated tumor immune tolerance is a major reason why tumor cells evade immune surveillance. The researchers found that HSP72, carried by tumor-derived exosomes (TDEs), suppresses the immunosuppressive effects of MDSCs by activating STAT3.
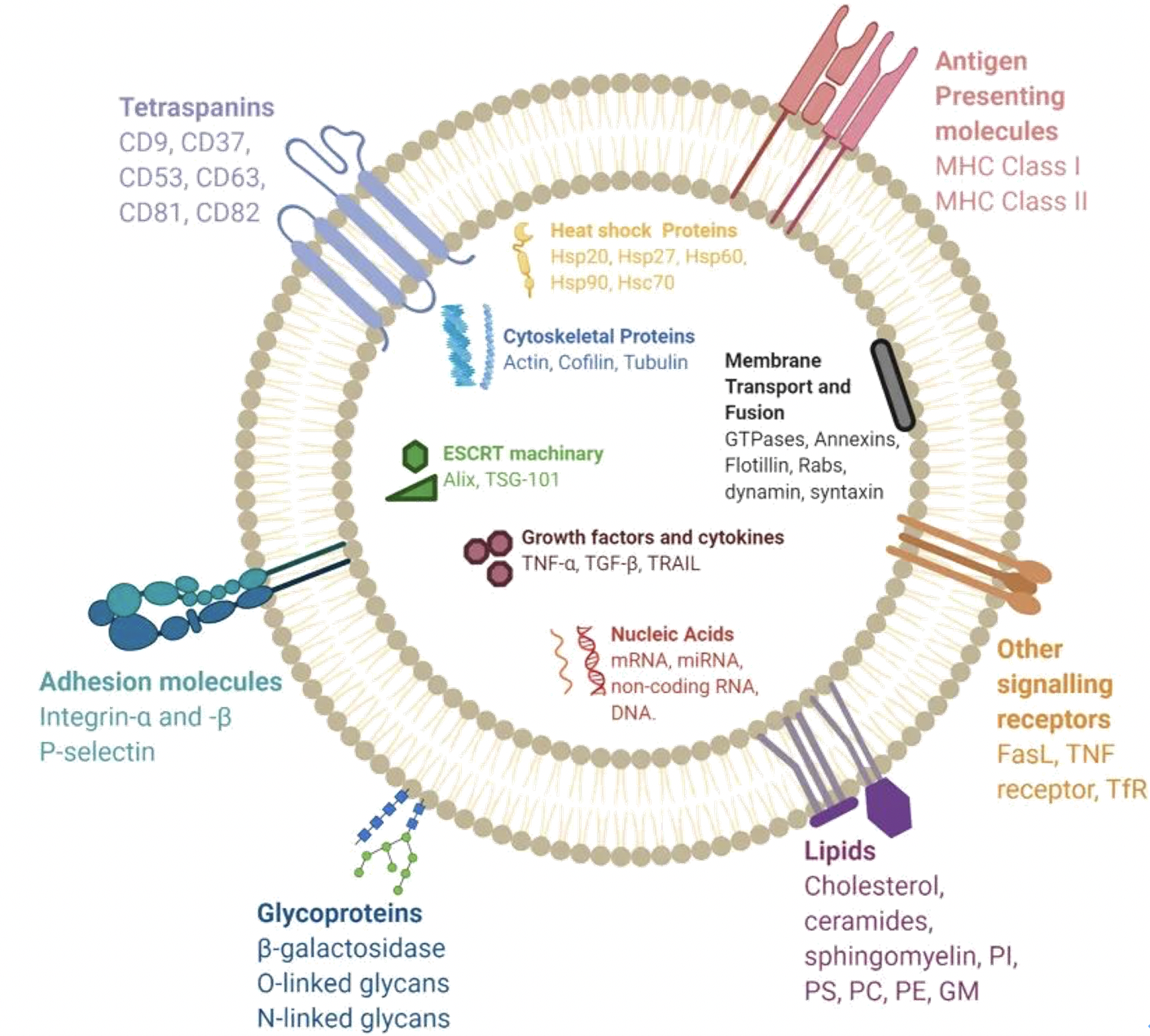
With the continuous development of sequencing technology, liquid biopsy techniques for cancer are becoming increasingly robust. Beyond CTCs and ctDNA, exosomes are emerging as a rising star that has been receiving increased attention, especially in oncology. With their widespread distribution, high content, and stable structure, they demonstrate immense advantages in tumor diagnosis (screening), monitoring, and treatment, such as novel biomarkers, targeted therapy, etc.
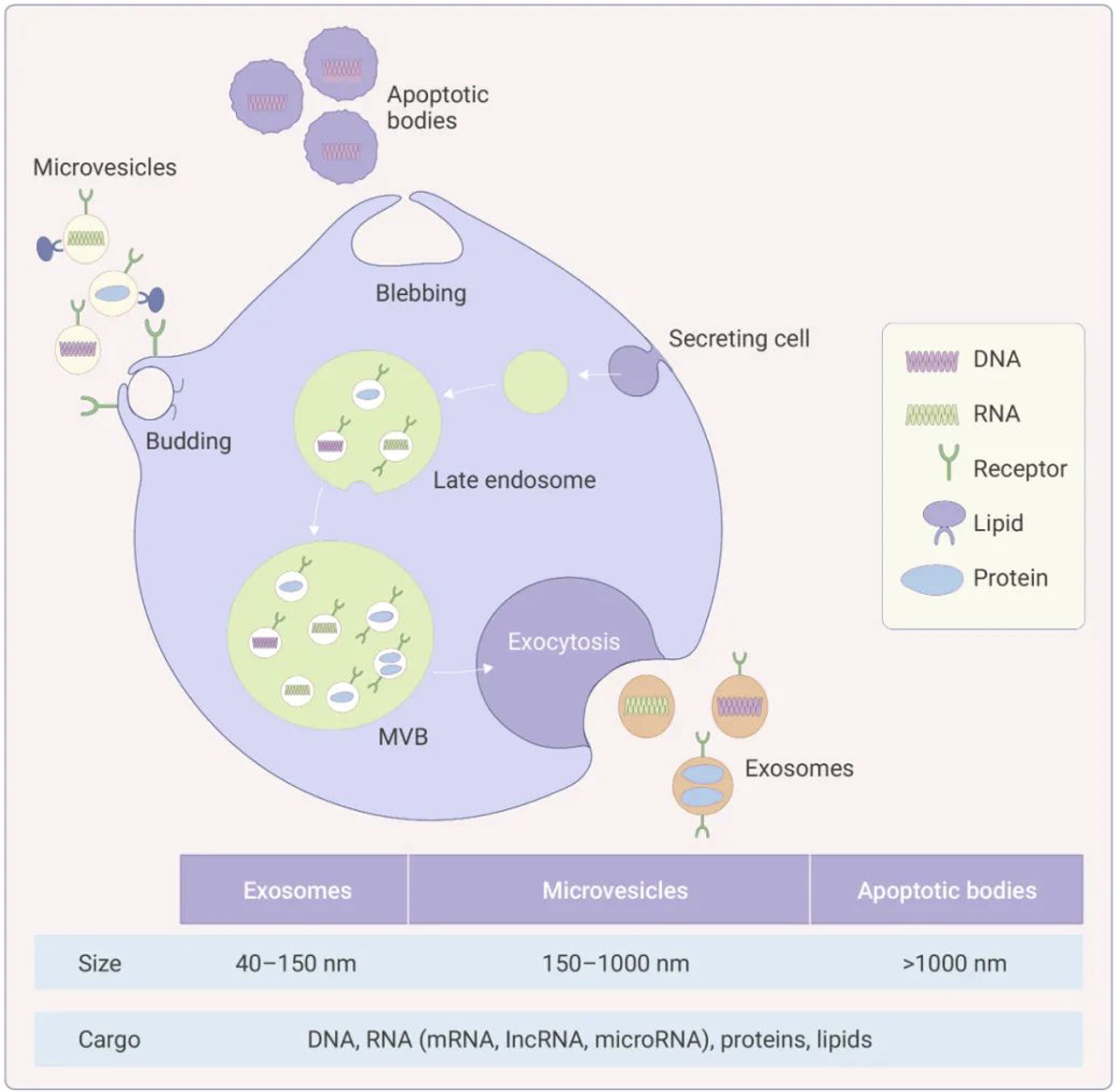
Exosomes were initially defined as membranous vesicles with a diameter of about 30~120 nm, released into the extracellular matrix following the fusion of multivesicular bodies (MVBs) with the cell membrane. Now, they particularly refer to disk-shaped vesicles with a diameter of 40-100 nm. Before 1983, exosomes were believed to be tools used by cells to excrete waste. It was later discovered that they can reach other cells or tissues through the circulatory system, carrying a large number of biomolecules from the parent cell to other cells, including proteins, nucleic acids (miRNAs, mRNAs, lncRNAs, circRNAs, DNA, etc.), and lipids. These contents endow exosomes with a variety of biological functions, such as intercellular information transfer, antigen presentation in immunity, and tumor growth and migration.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug type.
Exosome-mediated Tumor Immune-Evasion and Tolerance
Studies have found that tumor cells can produce a large number of exosomes, using these exosomes to transfer oncogenes to other cells, and alter their original environment to establish a conducive microenvironment for their own metastasis and colonization. Beyond this, they can additionally transmit signal factors capable of inhibiting the immune response against tumors, thus lowering the immunity of tumor patients, especially those in the late stage.
As we all know, issues of tumor immune evasion and tolerance persistently plague tumor researchers. Seen from this perspective, tumor-derived exosomes (TDEs) would appear to be one of the major factors that cannot be overlooked.
Exosome-Mediated Tumor Immune Tolerance
Immune tolerance is a primary reason for tumor cells to evade immune surveillance, and it is related to that the tumor-related antigens and immune suppressor molecules contained in the exosome-mediated immune tolerance, which can weaken the expression of the immune system. Tumor-derived exosomes (TDEs) can also deliver certain inhibitory signals, playing a negative regulatory role in the body's immune response process, thereby inducing immune tolerance in tumor cells. Studies have shown that the immune tolerance induced by TDEs is associated with their surface FasL. Fas is present on the surface of numerous cells and quickly increases its quantities when the body is exposed to external stimulations, inducing cell death through the Fas/FasL pathway to regulate immune responses and maintain immune tolerance.
In addition, Myeloid-derived suppressor cells (MDSCs) are a heterogeneous group of cells originating from the bone marrow and are the precursors of dendritic cells (DCs), macrophages, and/or granulocytes. They have significant abilities to suppress immune responses. Researchers found that HSP72 carried by tumor-derived exosomes (TDEs) inhibited the immune suppressive effect of MDSCs by activating STAT3, thereby achieving immune tolerance.
Exosome-Mediated Tumor Immune Escape
One of the reasons for tumor immune evasion is that proteins and RNAs that can promote cancer metastasis can utilize exosomes as escape carriers to avoid the body's immune response. Additionally, within the tumor microenvironment, exosomes can be transferred between tumor cells, immune cells, and stromal cells, serving as communicators to help tumor cells evade immune surveillance.
Furthermore, studies have shown that tumor-derived exosomes (TDEs) can act as pawns, substituting for tumor cells to take the onslaught of the immune system, aiding tumor cells in achieving immune escape. It can be said exosomes are quite "loyal".
Recent research found that melanoma cells secrete exosomes loaded with the protein PD-L1, which can directly bind with T cells, inhibiting their function and causing them to become "exhausted" before they can combat the cancer cells. This finding has emerged as a new insight in our understanding of tumor immune escape.
Exosomes and Tumor Immunotherapy
As most cells, including tumor cells and immune cells, can secrete exosomes, it has been found that exosomes derived from immune cells can inhibit the growth, proliferation, and metastasis of tumors.
Studies have found that exosomes secreted by Antigen-Presenting Cells (APCs) can stimulate the proliferation of T cells in vitro and induce anti-tumor immune responses in vivo. Exosomes secreted from tumor cells containing tumor antigens can also be cross-presented to cytotoxic T lymphocytes (CTLs) by APCs, thereby exerting a tumor-killing effect. Therefore, exosomes are currently being widely studied as a potentially significant tumor vaccine.
In addition, the melanoma-derived exosomes carrying PD-L1 protein mentioned above not only explain why the immune system of cancer patients is weakened, but more importantly, reveal why PD-1 targeted therapy is ineffective in 70% of melanoma patients. This has important implications for the current popular anti-PD-1/PD-L1 immunotherapy and is a significant step towards precision and personalized medicine.
Furthermore, based on the differences in exosomes derived from different cells, researchers have proposed methods to predict which cancer patient would respond to certain checkpoint inhibitors by detecting circulating exosomes. This would not only enable precise treatment but also allow tracking the effectiveness of these therapies.