Merck Initiates Phase 3 Clinical trial for PCSK9 Inhibitor MK-0616
August 25, 2023 -- Merck, also recognized as MSD outside of the United States and Canada, has today made public the launch of the company's Phase 3 clinical trials, labeled as CORALreef, for MK-0616, a potential oral proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor. The candidate is under investigation as a solution for adults struggling with hypercholesterolemia. This marks the debut of any Phase 3 clinical assessments for an oral PCSK9 inhibitor. The primary participants are currently enrolling in two Phase 3 trials that will determine the reduction in low-density lipoprotein (LDL) cholesterol: CORALreef Lipids and CORALreef HeFH. Further, Merck is set to initiate the Phase 3 cardiovascular outcomes trial, CORALreef Outcomes, by the closing months of 2023.
 PCSK9 has been recognized as a significant target for reducing LDL cholesterol levels. Yet, no oral PCSK9 inhibitors are in the market for physicians and patients. The Phase 3 CORALreef trials stem from impressive Phase 2b results unveiled at the ACC.23/WCC event. Here, MK-0616 showcased a significant decline in LDL cholesterol when pitched against a placebo at all dosage levels studied amid participants with hypercholesterolemia and a diverse range of atherosclerotic cardiovascular diseases (ASCVD) risk, inclusive of participants on high-dosage statin therapy. MK-0616 demonstrated a good tolerance level overall.
PCSK9 has been recognized as a significant target for reducing LDL cholesterol levels. Yet, no oral PCSK9 inhibitors are in the market for physicians and patients. The Phase 3 CORALreef trials stem from impressive Phase 2b results unveiled at the ACC.23/WCC event. Here, MK-0616 showcased a significant decline in LDL cholesterol when pitched against a placebo at all dosage levels studied amid participants with hypercholesterolemia and a diverse range of atherosclerotic cardiovascular diseases (ASCVD) risk, inclusive of participants on high-dosage statin therapy. MK-0616 demonstrated a good tolerance level overall.
"High LDL cholesterol is a key contributor to cardiovascular disease, which is the primary cause of mortality globally. Despite the existence of multiple effective lipid-reduction treatments, a large number of individuals across the world fail to achieve and maintain desired LDL cholesterol management targets, exposing them to the danger of cardiac incidents," explained Dr. Marc Sabatine, Head of the Thrombolysis in Myocardial Infarction (TIMI) Study Group, Lewis Dexter, MD, Honored Chair in Cardiovascular Medicine at Brigham and Women’s Hospital, and a Professor of Medicine at Harvard Medical School. "MK-0616 demonstrated significant reduction in LDL cholesterol in the Phase 2 trial, offering a distinct feature of being a once-daily medication. Given its unique mechanism of action among PCSK9 inhibitors, MK-0616 may afford a critical solution for patients in managing hypercholesterolemia."
"The launching of an all-embracing Phase 3 program marks a significant turning point in our journey to provide a highly potent oral drug, accessible to a wide population segment which could significantly increase the number of individuals achieving their LDL treatment objectives,” stated Dr. Joerg Koglin, high-ranking vice president, international clinical development, Merck Research Laboratories. "In recognition of the considerable racial, ethnic and gender imbalance in cardiovascular treatment, Merck is actively taking steps to involve potential candidates from communities that have typically been underrepresented in clinical trials of this nature.”
About PCSK9 and MK-0616
PCSK9 has a pivotal function in maintaining cholesterol balance by controlling the measures of the LDL receptor that facilitates cholesterol ingestion into cells. By obstructing PCSK9, it stops its collaboration with LDL receptors. This leads to an increase in the quantity of LDL receptors present on the cellular surface to extract LDL cholesterol from the bloodstream.
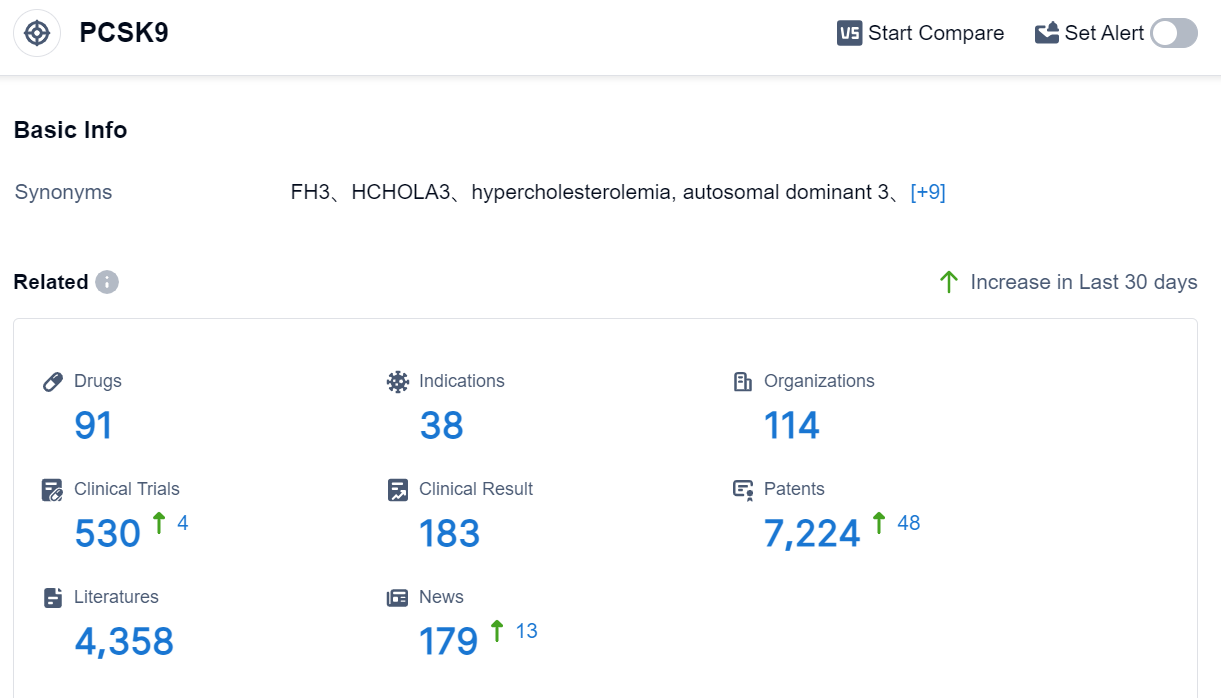
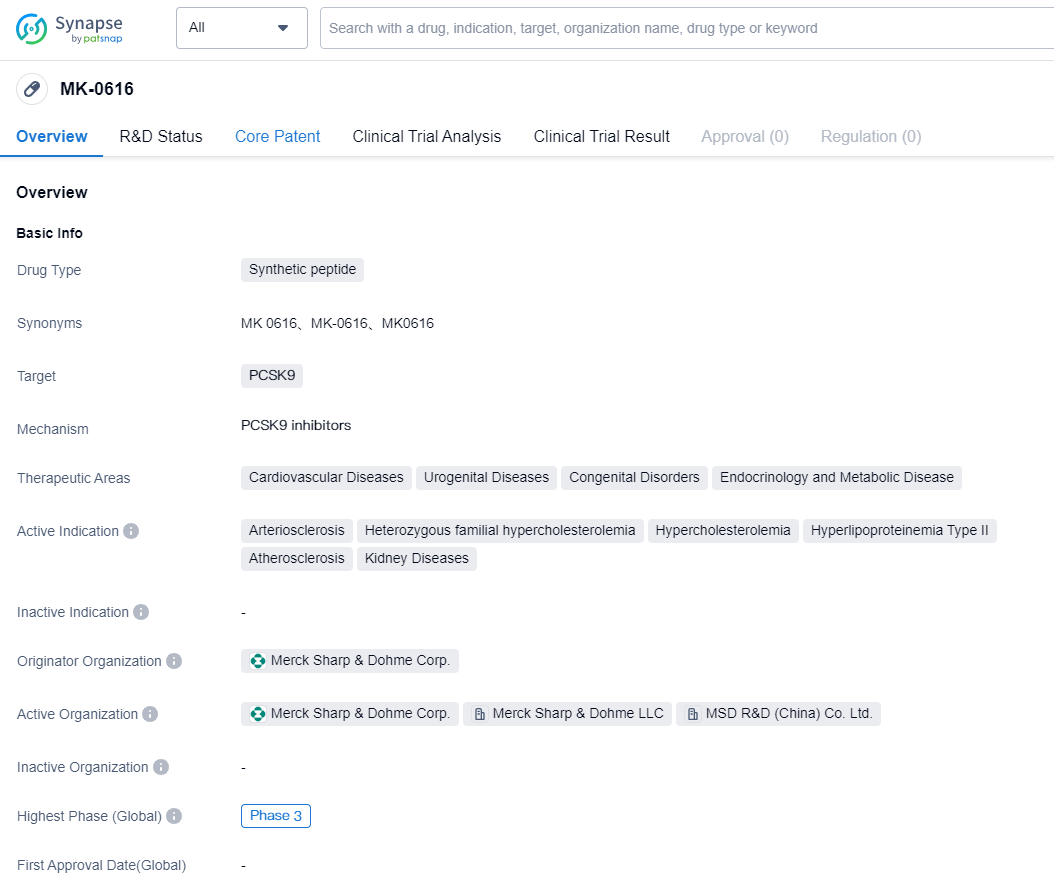
According to the information disclosed by the Synapse Database (Click on the image below for direct access to the latest R&D progress on PCSK9 target drugs, indications, research institutions, clinical trials, and more.), as of August 29, 2023, there are a total of 91 drugs under research for PCSK9 target, covering 38 indications, with 114 research institutions involved, related to 530 clinical trials and as many as 7224 patents. The dyslipidemia market experienced a steep downturn following the end of patent protection for statins and ezetimibe, and new drugs have yet to fill the vacancy left by this loss. Challenges such as PCSK9 reimbursement problems, the introduction of generics for Vascepa, the ineffective result of CETP inhibitors, and uncertainties surrounding the cardiovascular benefits of reducing triglycerides have exacerbated the situation. However, the market may be primed for a resurgence, courtesy of innovative strategies. Predictions indicate potential growth to $11.2bn by 2032, although this will hinge on an array of determinants related to payers and physicians.