Phase 3 Trials of Datopotamab Deruxtecan in Advanced Non-Squamous NSCLC Begin
The initial participants have received treatment in three worldwide, randomized phase 3 studies assessing the effectiveness and safety of datopotamab deruxtecan (Dato-DXd) combinations for individuals with locally advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Datopotamab deruxtecan is a targeted TROP2-directed antibody-drug conjugate (ADC) that has been developed by Daiichi Sankyo (TSE: 4568) and is currently in joint development with AstraZeneca (LSE/STO/Nasdaq: AZN). The TROPION-Lung10 study is investigating the efficacy of datopotamab deruxtecan combined with rilvegostomig, a bispecific antibody targeting PD-1/TIGIT from AstraZeneca, versus pembrolizumab, in patients with untreated, advanced or metastatic nonsquamous NSCLC exhibiting high PD-L1 expression (tumor cells [TC] ≥ 50%) and lacking actionable genomic alterations.
Meanwhile, TROPION-Lung14 is assessing the combination of datopotamab deruxtecan and osimertinib, AstraZeneca’s EGFR tyrosine kinase inhibitor (TKI), compared to osimertinib alone in individuals with previously untreated, locally advanced, or metastatic EGFR-mutated nonsquamous NSCLC. TROPION-Lung15 focuses on either the combination of datopotamab deruxtecan with or without osimertinib, or platinum-based double chemotherapy in patients with locally advanced or metastatic nonsquamous EGFR-mutant NSCLC whose condition has progressed after receiving osimertinib.
“These three studies in patients with high PD-L1 expression or EGFR mutations in nonsquamous non-small cell lung cancer are vital for understanding the potential utility of datopotamab deruxtecan across various lines and types of lung cancer treatment,” stated Mark Rutstein, MD, Global Head of Oncology Clinical Development at Daiichi Sankyo. “Our expanding TROPION clinical initiative now encompasses seven phase 3 trials, showcasing our dedication to fully exploring the capabilities of datopotamab deruxtecan in lung cancer therapy.”
“Clinical findings indicate that the pairing of an antibody-drug conjugate with a targeted treatment or bispecific immunotherapy may yield more robust and sustained tumor responses in various cancers, including lung cancer,” remarked Cristian Massacesi, MD, Chief Medical Officer and Oncology Chief Development Officer at AstraZeneca. “The commencement of these three additional phase 3 trials featuring datopotamab deruxtecan alongside our drugs, osimertinib and rilvegostomig, demonstrates our commitment to investigating potential synergies within our oncology portfolio and the overall utility and combinability of this TROP2-targeted ADC across diverse non-small cell lung cancer contexts.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
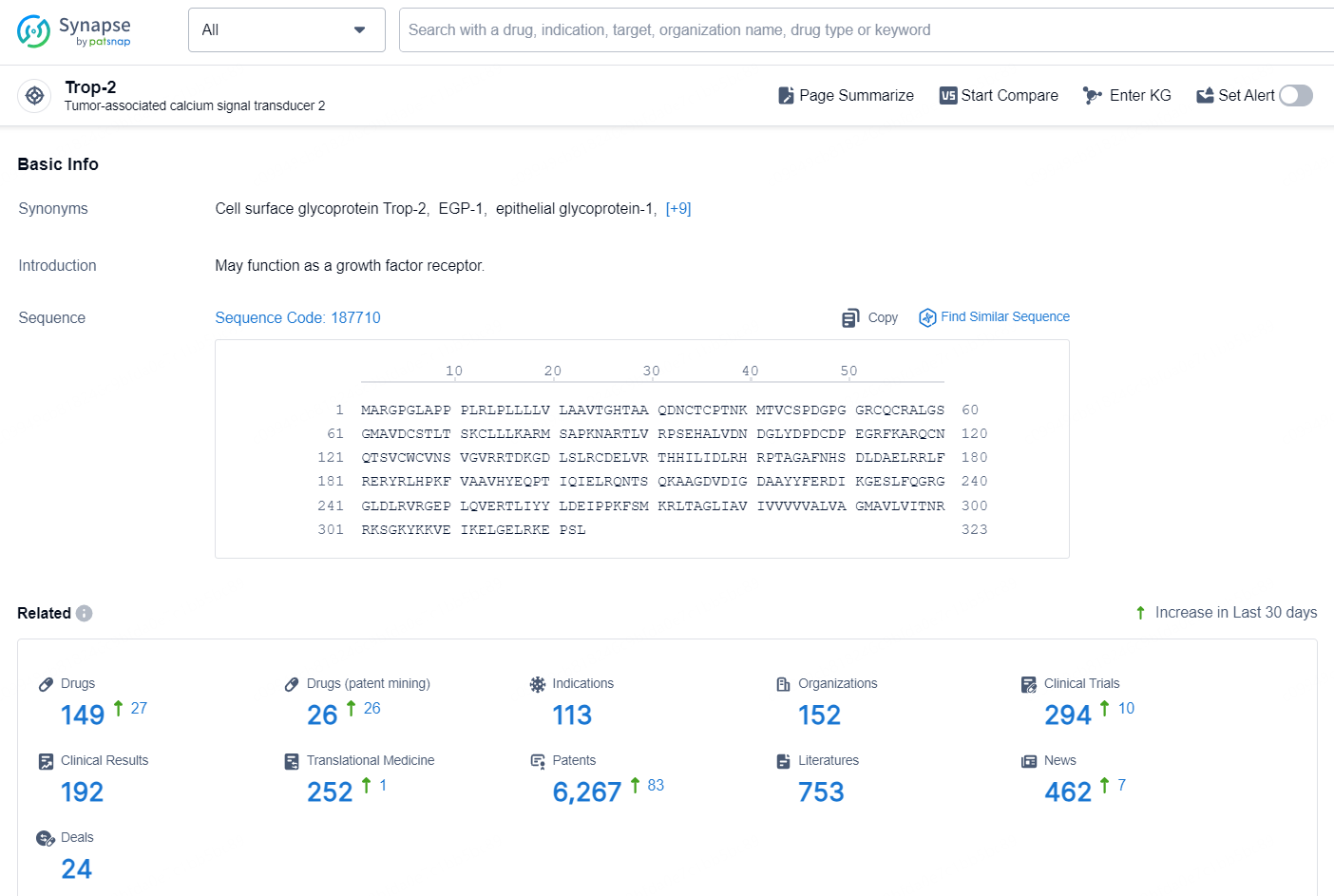
According to the data provided by the Synapse Chemical, As of October 31, 2024, there are 149 investigational drugs for the Trop-2 target, including 26 indications, 113 R&D institutions involved, with related clinical trial reaching 152, and as many as 6267 patents.
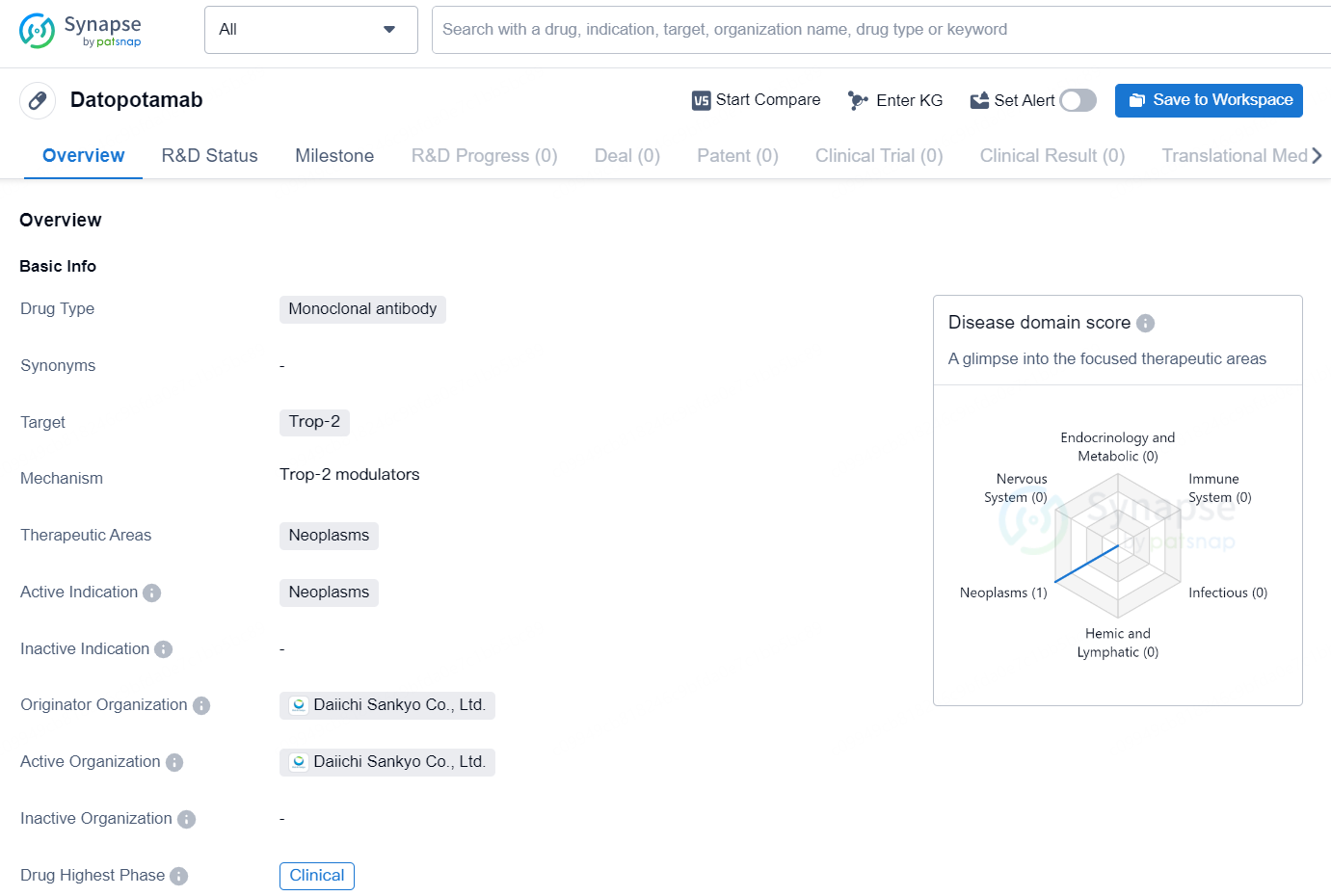
Datopotamab is a monoclonal antibody drug that targets the protein Trop-2 and is primarily intended for the treatment of neoplasms. Neoplasms, also known as tumors, are abnormal growths of tissue that can be benign or malignant. The drug is developed by Daiichi Sankyo Co., Ltd., a global pharmaceutical organization.