Exploring Apixaban: A Global Perspective on Its Therapeutic Applications and Synthesis Advancements
Apixaban is a small molecule drug that targets factor Xa and has been approved for the treatment of various therapeutic areas, including Nervous System Diseases, Cardiovascular Diseases, Respiratory Diseases, and Neoplasms. Its active indications include recurrent deep vein thrombosis, thrombosis, ischemic stroke, systemic embolism, atrial fibrillation, embolism, pulmonary embolism, stroke, venous thromboembolism, venous thrombosis, and neoplasms.
The drug was first approved in 2011 in the European Union, Iceland, Liechtenstein, and Norway. Its originator organization is Bristol Myers Squibb Co. The highest phase of approval for Apixaban is 'Approved' both globally and in China. Regulatory processes for the drug include Priority Review and Fast Track.
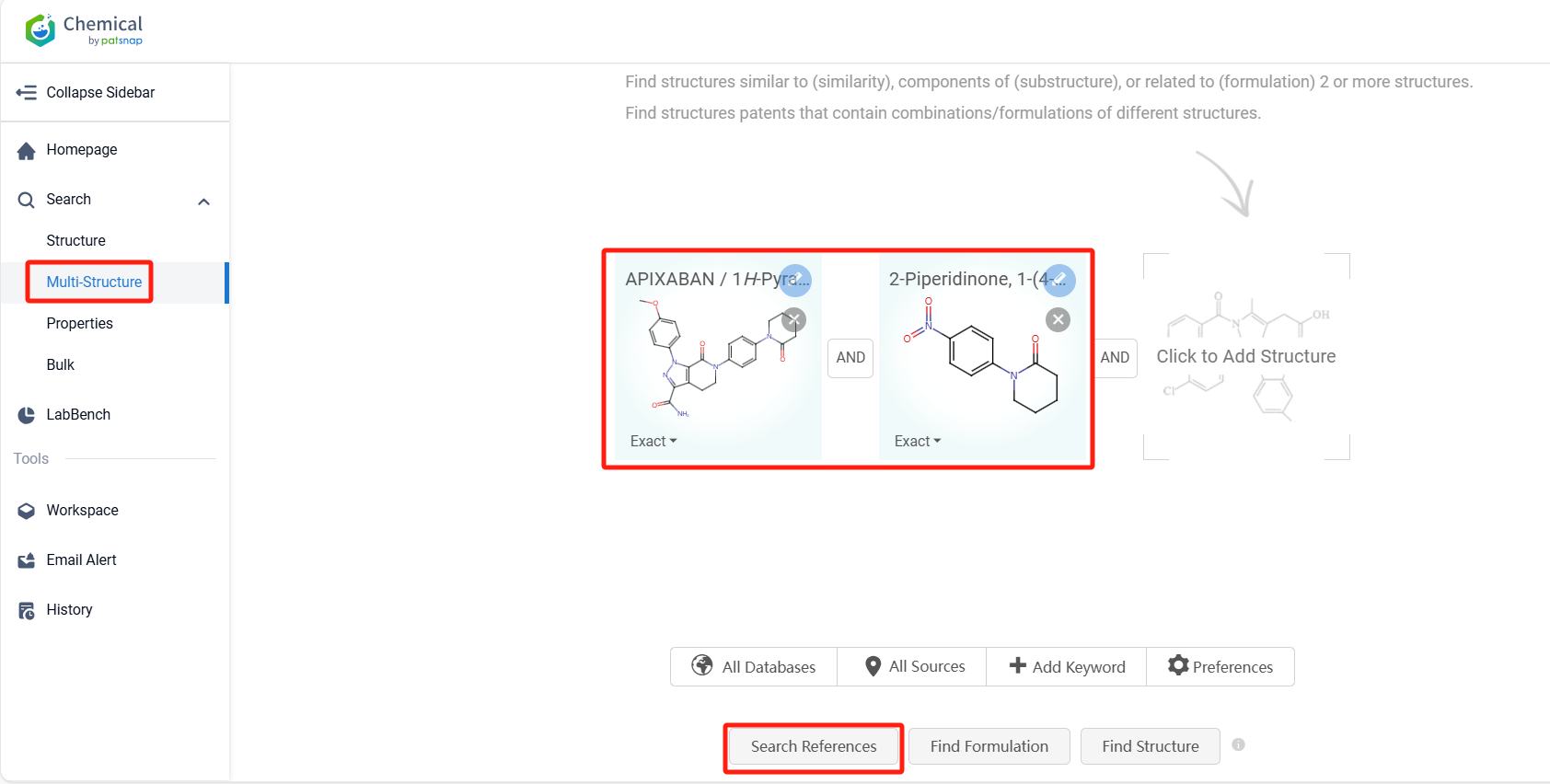
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Apixaban. click on search references, and you can query the relevant literature.
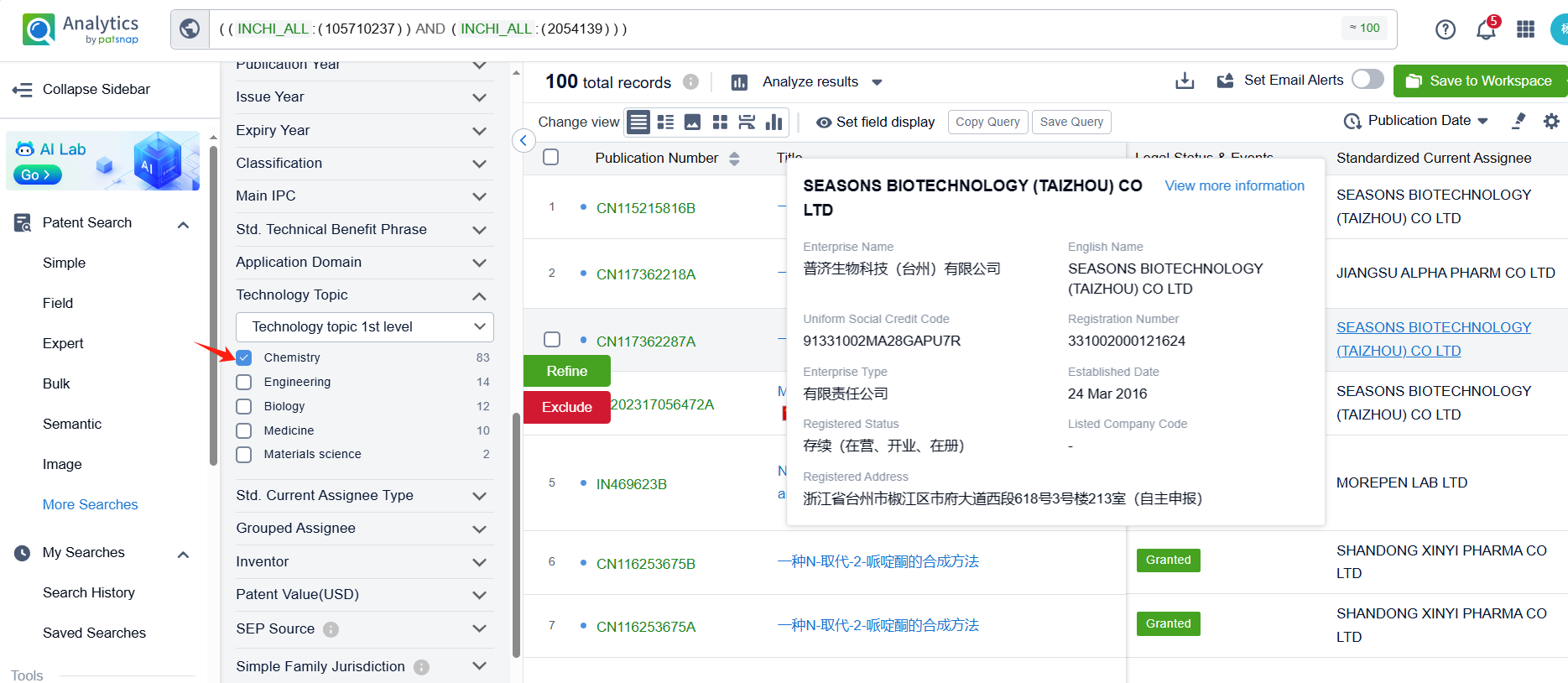
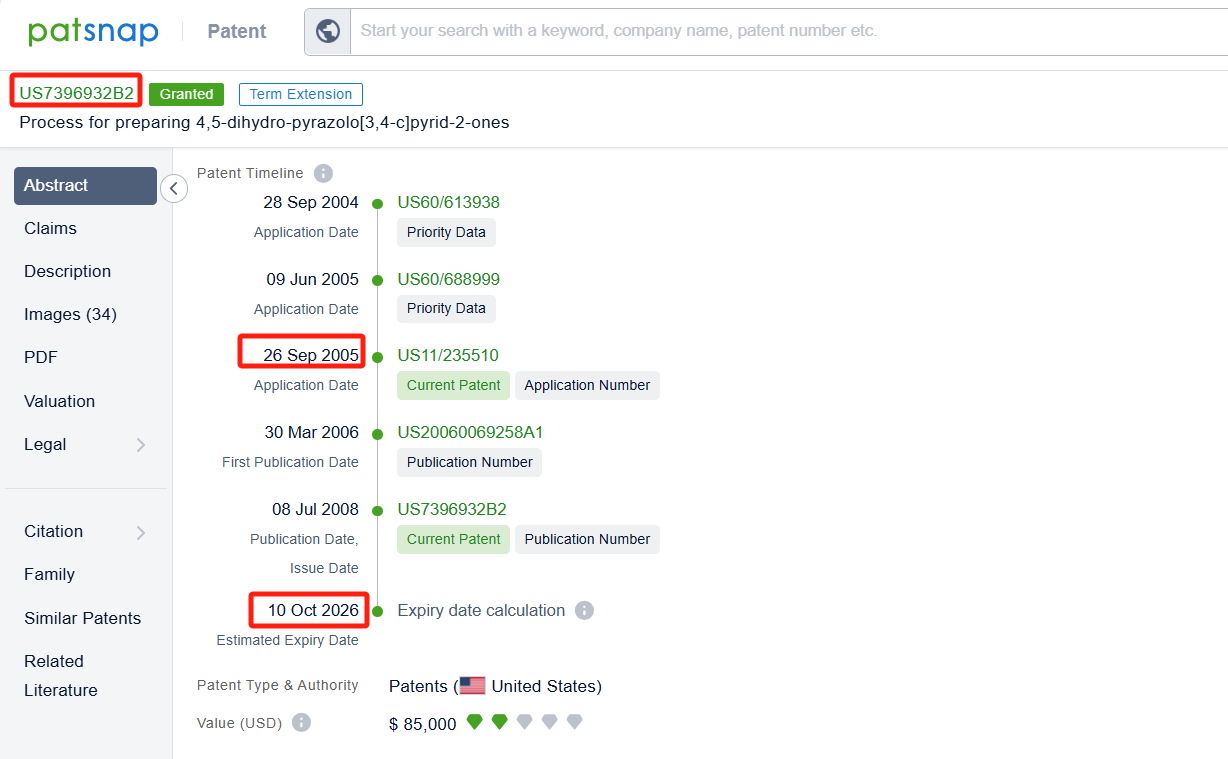
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Apixaban process route by Bristol Myers Squibb Co. and other companies,Such as Bristol Myers Squibb Co.'s patent US7396932B2 (application date 20050926, publication date 20060330) describes a process for preparing 4,5-dihydro-pyrazolo[3,4-c]pyrid-2-ones. The patent was granted on June 8, 2008, with the patent estimated expiry date being October 10, 2026. Unichem Laboratories Ltd.'s patent US20180099963A1(application date 20170906, publication date 20180412) provides a process to make Apixaban from Aniline and intermediates with high yield and under milder conditions. The patent was granted on November 16, 2018, with an expiration date of September 1, 2035. Additionally, Jiangsu Alpha Pharmaceutical Co., Ltd.'s patent CN117362218A (application date 20231024, publication date 20240109) provides a new synthesis process of apixaban key intermediate. By improving the reaction route and using a specific catalyst, the cost of reaction raw materials can be reduced and the reaction yield can be improved.

Apixaban holds significance in the pharmaceutical industry due to its approval for multiple therapeutic areas, as well as its fast-track regulatory status. Its targeting of factor Xa makes it a valuable asset in the treatment of various cardiovascular and thrombotic conditions. Additionally, its approval in multiple countries, including the EU and China, highlights its international success. The drug's approval in 2011 also suggests that it has been in the market for a substantial period, indicating its established presence and potential market impact.
In conclusion, Apixaban is a small molecule drug that has demonstrated efficacy in treating a wide range of therapeutic areas and is approved in multiple countries. Its approval status, target specificity, and regulatory designations make it a prominent player in the pharmaceutical industry's landscape.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.