Exploring Camrelizumab's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Camrelizumab's R&D Progress
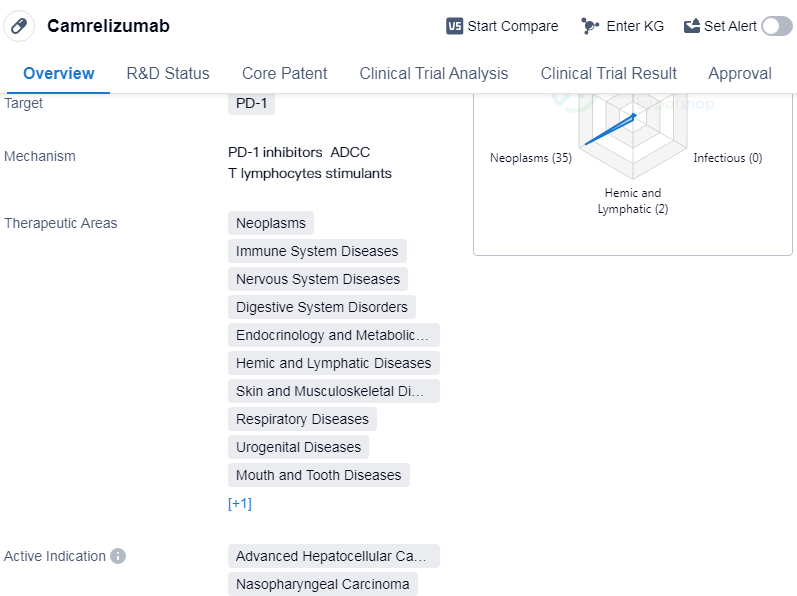
Camrelizumab is a monoclonal antibody drug that targets PD-1, a protein involved in regulating the immune system. It has been approved for use in various therapeutic areas, including neoplasms (tumors), immune system diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, hemic and lymphatic diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, mouth and tooth diseases, and otorhinolaryngologic diseases.
The drug has shown efficacy in treating advanced hepatocellular carcinoma, nasopharyngeal carcinoma, esophageal squamous cell carcinoma, non-small cell lung cancer, Hodgkin's lymphoma, etc.
Camrelizumab was developed by Jiangsu Hengrui Pharmaceuticals Co., Ltd., and it received its first approval in China in May 2019. It has also received approvals in other countries. The drug has undergone priority review and has been designated as a breakthrough therapy and an orphan drug, indicating its potential to address unmet medical needs.
As a monoclonal antibody targeting PD-1, camrelizumab works by blocking the interaction between PD-1 and its ligands, PD-L1 and PD-L2. This blockade helps to restore the immune system's ability to recognize and attack cancer cells. By targeting PD-1, camrelizumab has shown promise in improving outcomes for patients with various types of cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Camrelizumab: PD-1 inhibitors, ADCCT Lymphocytes Stimulants
PD-1 inhibitors are a type of medication that work by blocking the programmed cell death protein 1 (PD-1) receptor on immune cells. PD-1 is a protein found on the surface of T cells, which are a type of lymphocyte involved in the immune response. When PD-1 binds to its ligands, it sends signals that suppress the immune response, helping to prevent excessive immune activation and tissue damage. However, some cancer cells can exploit this mechanism to evade the immune system.
PD-1 inhibitors, such as pembrolizumab and nivolumab, bind to PD-1 receptors and prevent them from interacting with their ligands. This blockade releases the "brakes" on the immune system, allowing T cells to recognize and attack cancer cells more effectively. By inhibiting PD-1, these drugs can enhance the immune response against cancer and potentially improve treatment outcomes.
ADCCT, on the other hand, does not correspond to any recognized term or acronym in the field of biomedicine or general understanding. Without further context, it is not possible to provide a specific explanation for this term.
Lymphocytes are a type of white blood cell that play a crucial role in the immune system's defense against infections and diseases. They are produced in the bone marrow and can be found in various tissues throughout the body, including the lymph nodes, spleen, and thymus. Lymphocytes are responsible for recognizing and attacking foreign substances, such as viruses, bacteria, and cancer cells.
Stimulants, in a general sense, refer to substances or agents that increase physiological or nervous system activity. They can enhance alertness, attention, and energy levels. However, without additional context, it is not clear how stimulants are specifically related to lymphocytes in this case.
Drug Target R&D Trends for Camrelizumab
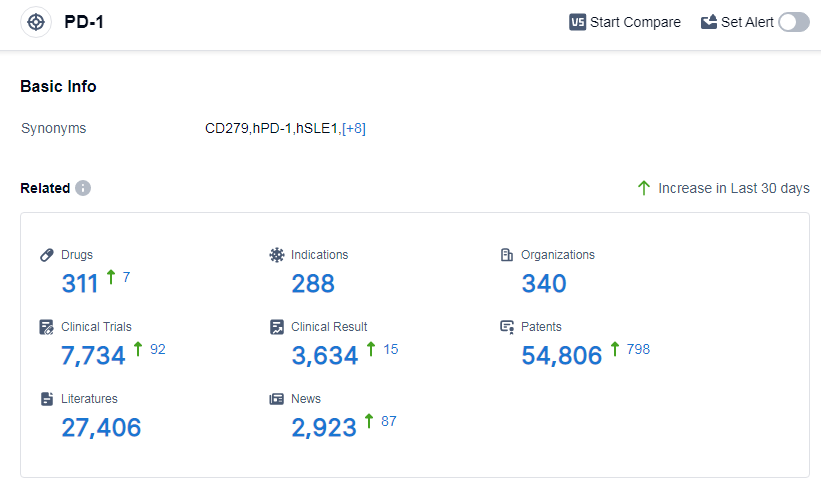
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 311 PD-1 drugs worldwide, from 340 organizations, covering 288 indications, and conducting 7734 clinical trials.
The analysis of the target PD-1 reveals a highly competitive landscape with multiple companies actively involved in research and development. Akeso, Inc., Bristol Myers Squibb Co., and Merck & Co., Inc. are the leading companies in terms of drug count and development stages. PD-1 inhibitors have shown promising results in various indications, including Hodgkin's lymphoma, non-small cell lung cancer, melanoma, and esophageal squamous cell carcinoma. Monoclonal antibodies are the most prevalent drug type, followed by bispecific antibodies and biosimilars. China is at the forefront of PD-1 research, with a significant number of approved drugs and ongoing development. The United States, the European Union, and other countries also contribute significantly to PD-1 research. Overall, the target PD-1 presents a competitive landscape with diverse drug types and indications, indicating a promising future for PD-1 inhibitors in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Camrelizumab is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of multiple types of cancers. It has shown efficacy in various therapeutic areas and has received approvals in China and other countries. Its development by Jiangsu Hengrui Pharmaceuticals Co., Ltd., and its designations as a breakthrough therapy and an orphan drug highlight its potential in addressing unmet medical needs.