Exploring Ciprofloxacin Hydrochloride's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Ciprofloxacin Hydrochloride's R&D Progress
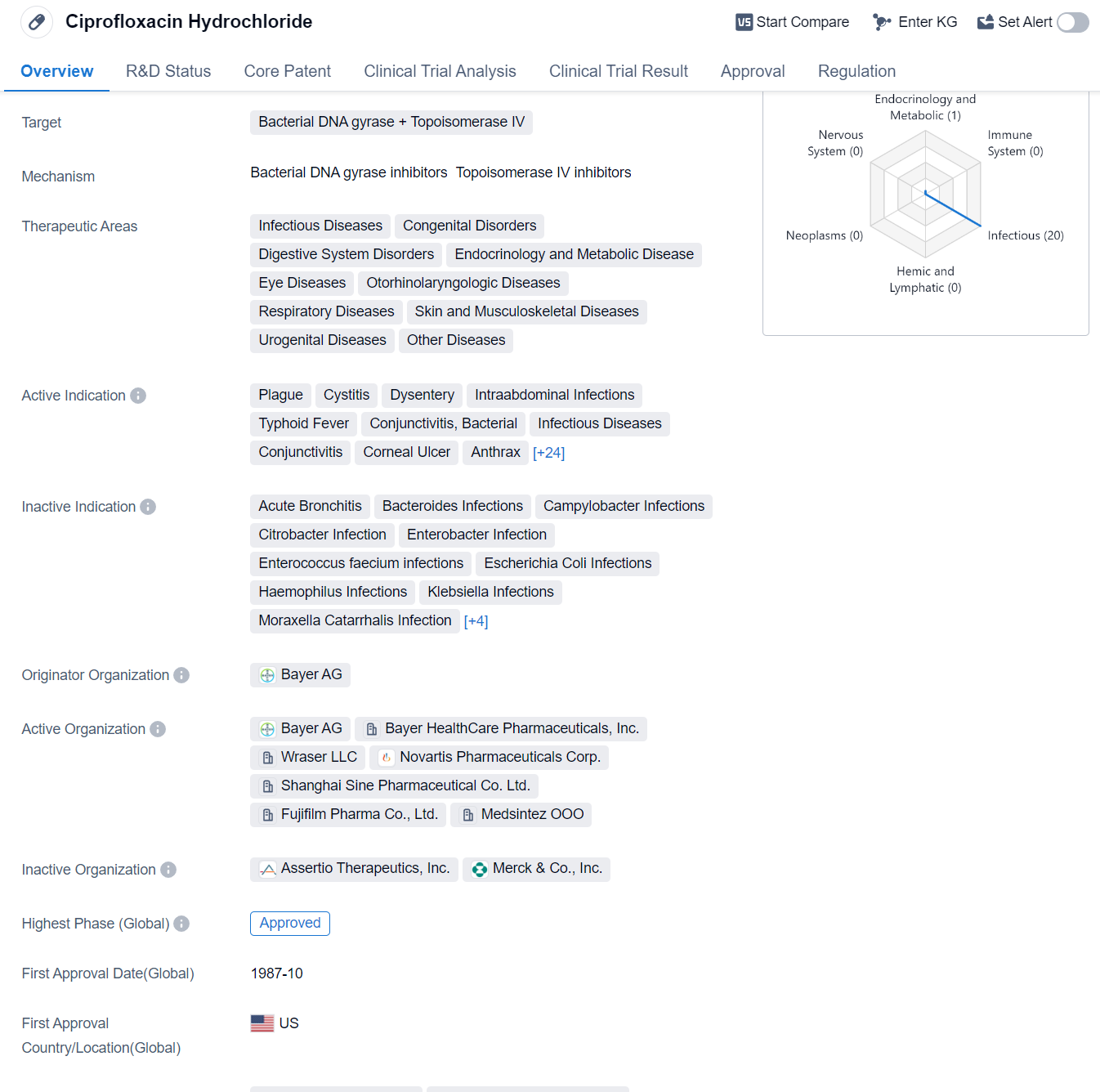
Ciprofloxacin Hydrochloride is a small molecule drug that targets bacterial DNA gyrase and Topoisomerase IV. It has been approved for various therapeutic areas including infectious diseases, congenital disorders, digestive system disorders, endocrinology and metabolic disease, eye diseases, otorhinolaryngologic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
The drug has shown efficacy in treating a wide range of infections caused by bacteria. Some of the active indications for Ciprofloxacin Hydrochloride include plague, cystitis, dysentery, intraabdominal infections, typhoid fever, conjunctivitis (bacterial), infectious diseases, conjunctivitis, corneal ulcer, anthrax, bacterial infections, cystic fibrosis, diarrhea, gastrointestinal diseases, gonorrhea, gram-negative bacterial infections, meningococcal infections, orchitis, otitis externa, otitis media, pelvic inflammatory disease, pneumonia (bacterial), chronic obstructive pulmonary disease, pyelonephritis, respiratory tract infections, skin diseases (bacterial), urethritis, bone and joint infections, inhalation anthrax, lower respiratory tract infections, prostatitis, sinusitis, skin and skin structure infections, and urinary tract infections.
The drug was originally developed by Bayer AG and received its first approval in the United States in October 1987. It is classified as an orphan drug, indicating that it is used to treat rare diseases or conditions. Ciprofloxacin Hydrochloride has also received approvals in other countries, making it a globally recognized medication.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ciprofloxacin Hydrochloride: Bacterial DNA gyrase inhibitor and Topoisomerase Ⅳ inhibitor
Bacterial DNA gyrase inhibitors and Topoisomerase IV inhibitors are types of drugs that target specific enzymes involved in DNA replication and repair in bacteria. These enzymes, DNA gyrase and Topoisomerase IV, are essential for bacterial cell division and survival.
Bacterial DNA gyrase inhibitors are drugs that specifically inhibit the activity of DNA gyrase, an enzyme responsible for introducing negative supercoils into bacterial DNA. By inhibiting DNA gyrase, these drugs interfere with the normal DNA replication process in bacteria, leading to the inhibition of bacterial growth and ultimately bacterial cell death.
Topoisomerase IV inhibitors, on the other hand, target another enzyme called Topoisomerase IV, which is involved in the separation of replicated DNA strands during bacterial cell division. By inhibiting the activity of Topoisomerase IV, these drugs prevent the proper separation of DNA strands, leading to the formation of abnormal DNA structures and the inhibition of bacterial cell division.
Both types of inhibitors are commonly used in the treatment of bacterial infections. They belong to a class of antibiotics known as fluoroquinolones, which are effective against a wide range of bacterial pathogens. These drugs are particularly useful in treating respiratory tract infections, urinary tract infections, and certain types of sexually transmitted infections caused by susceptible bacteria.
It is important to note that these drugs specifically target bacterial enzymes and do not have the same effect on human cells. This selectivity allows for the effective treatment of bacterial infections while minimizing harm to the patient. However, it is crucial to use these drugs judiciously to prevent the development of antibiotic resistance in bacteria.
Drug Target R&D Trends for Ciprofloxacin Hydrochloride
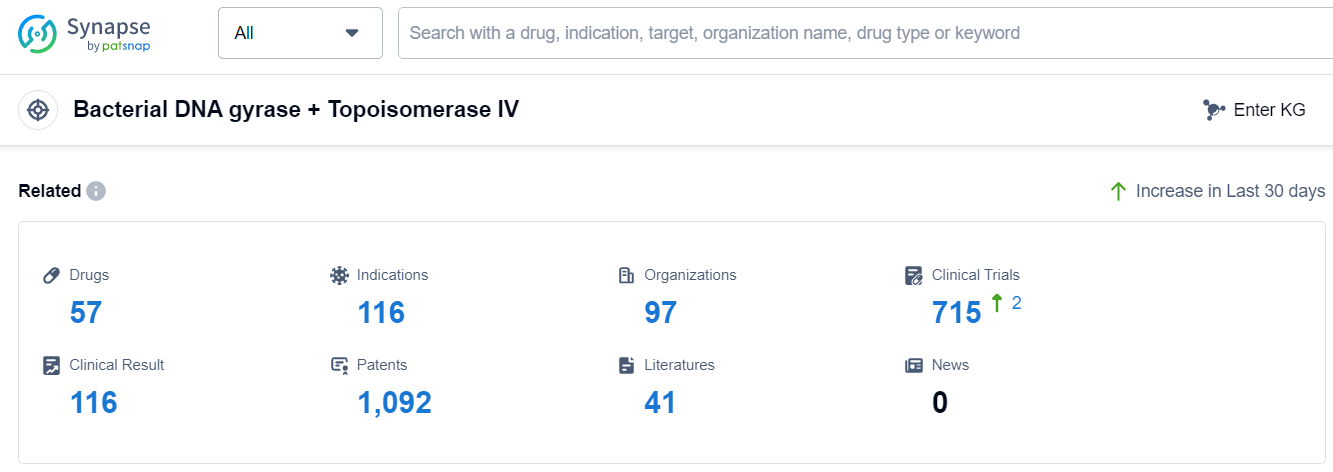
The analysis of the target Bacterial DNA gyrase + Topoisomerase IV reveals a competitive landscape with multiple companies actively involved in research and development. Novartis AG, Bayer AG, FUJIFILM Holdings Corp., Alcon AG, Taisho Pharmaceutical Holdings Co., Ltd., and Daiichi Sankyo Co., Ltd. are among the companies with the highest stage of development for this target. The approved indications for drugs targeting this target include Bacterial Infections, Respiratory Tract Infections, Urinary Tract Infections, and Infectious Diseases, among others. Small molecule drugs are progressing rapidly in the development of drugs for this target. China, Japan, and the United States are leading in terms of the number of drugs in the approved stage. Other countries such as the European Union, Germany, South Korea, Canada, South Africa, Brazil, Mexico, France, Thailand, Uruguay, Taiwan Province, United Kingdom, India, Spain, Poland, Russia, and Chile are also making progress in the development of drugs targeting this specific target. Overall, the target Bacterial DNA gyrase + Topoisomerase IV presents a competitive landscape with potential for future development and innovation in the pharmaceutical industry.
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 57 Bacterial DNA gyrase + Topoisomerase IV drugs worldwide, from 97 organizations, covering 116 indications, and conducting 715 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Ciprofloxacin Hydrochloride is a small molecule drug that targets bacterial DNA gyrase and Topoisomerase IV. It has been approved for various therapeutic areas and is effective in treating a wide range of bacterial infections. Developed by Bayer AG, the drug received its first approval in the United States in 1987 and is classified as an orphan drug. Its global approvals highlight its significance in the pharmaceutical industry.