Exploring Disitamab Vedotin's R&D successes and its clinical results at the 2024 ASCO_GU
For HER2 positive muscle-invasive bladder cancer (MIBC), the efficacy of cisplatin-based NAC is unsatisfied, and adverse reactions are inevitable or even intolerable. Disitamab vedotin is an antibody-drug conjugate composed of an anti-HER2 antibody disitamab and MMAE payload. Multiple clinical studies have confirmed that promising activity in HER2-positive locally advanced or mUC patients. Recently, the 2024 ASCO_GU updated the clinical data of disitamab vedotin, and toripalimab as a novel neoadjuvant treatment combination in patients with HER2 positive locally advanced MIBC.
Disitamab Vedotin's R&D Progress
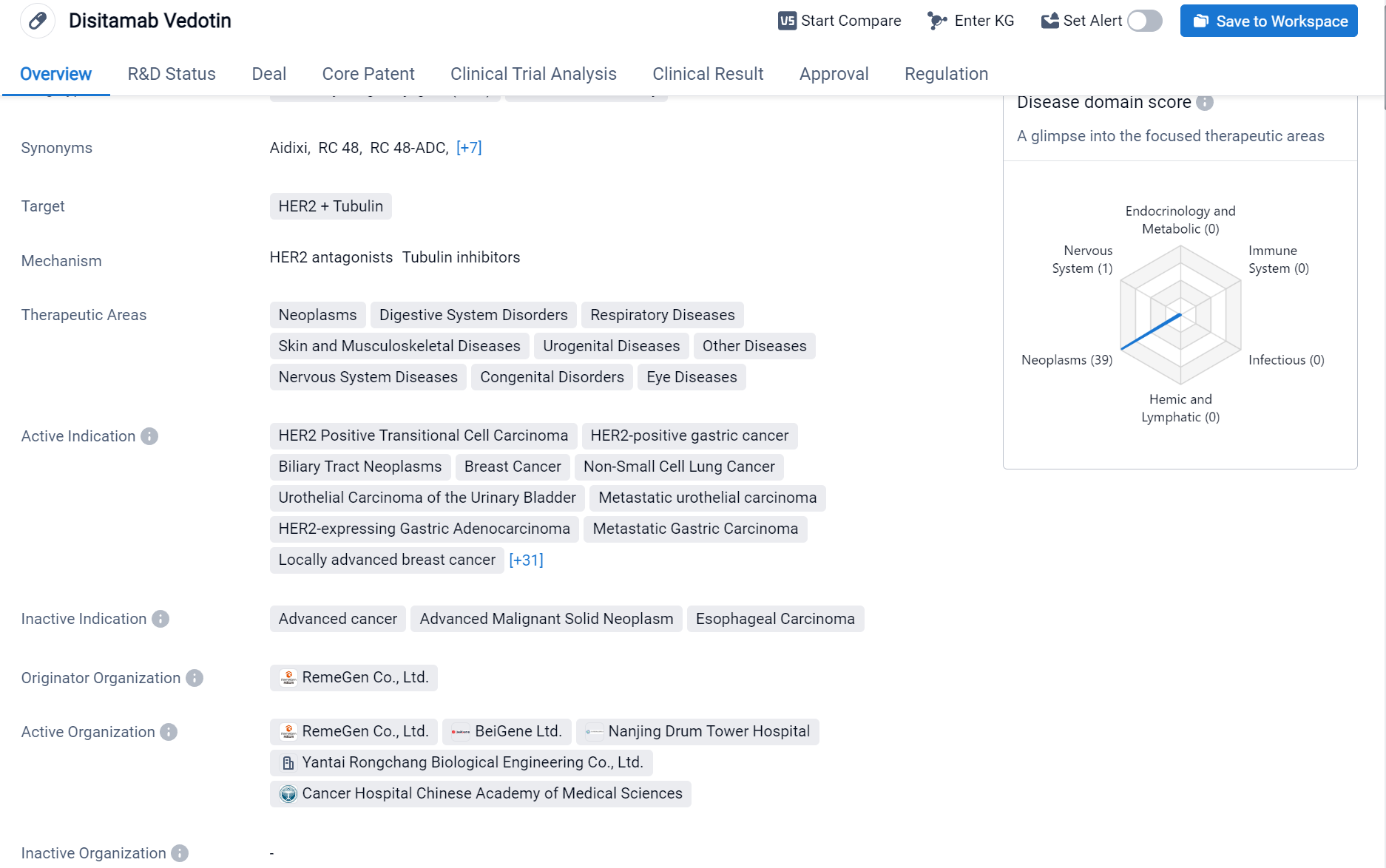
Disitamab Vedotin is an antibody drug conjugate (ADC) and monoclonal antibody that targets HER2 and Tubulin. It has been approved for various therapeutic areas including neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, other diseases, nervous system diseases, congenital disorders, eye diseases.
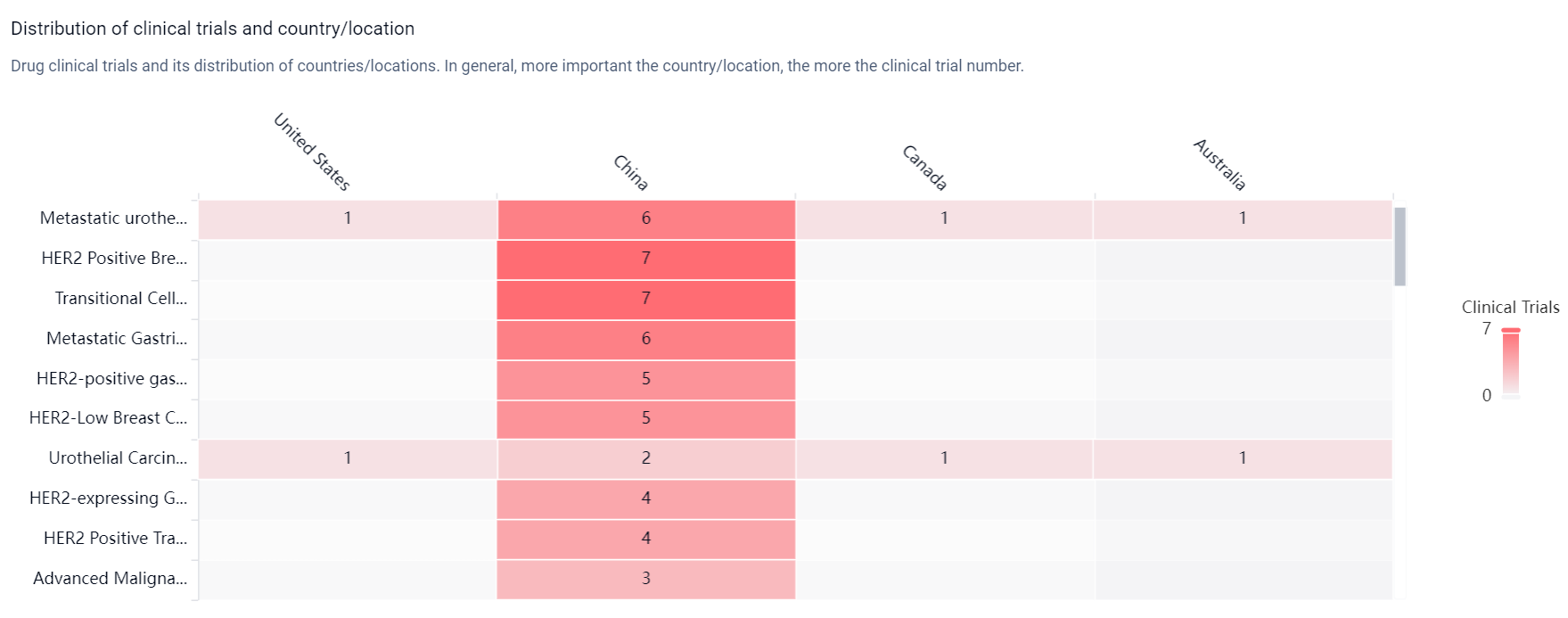
According to the Patsnap Synapse, Disitamab Vedotin was developed by RemeGen Co., Ltd. and has received approvals in China. It has also undergone priority review, conditional marketing approval, fast track designation, orphan drug status, and breakthrough therapy designation. The drug's highest phase of development is approved globally. And the clinical trial distributions for Disitamab Vedotin are primarily in the United States, China and Canada. The key indication is Metastatic urothelial carcinoma.
Detailed Clinical Result of Disitamab Vedotin
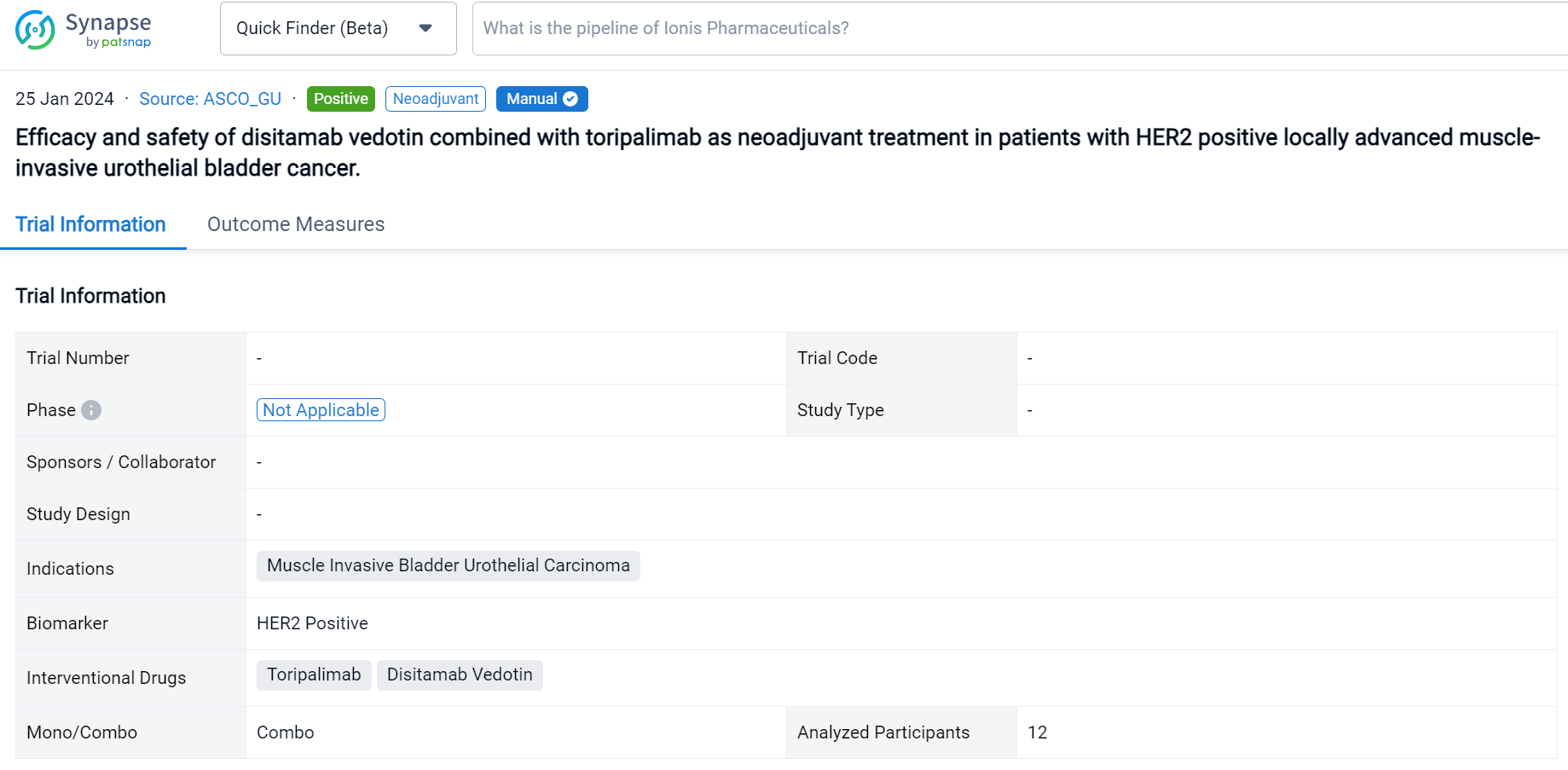
This trial was aimed to to evaluate the safety and efficacy of disitamab vedotin, and toripalimab as a novel neoadjuvant treatment combination in patients with HER2 positive locally advanced MIBC.
In this study, platinum intolerance and pathological andimaging diagnosed cT2-4bN0M0 MIBC patients with HER2 positive (HER2 IHC2+ or HER2 IHC3+) received 3 cycles toripalimab (200mg, iv, D1, Q2W) combined with disitamab vedotin (2mg/kg, iv, D1, Q2W). The primary endpoint was pCR, and the secondary endpoints included PFS, OS, and safety.
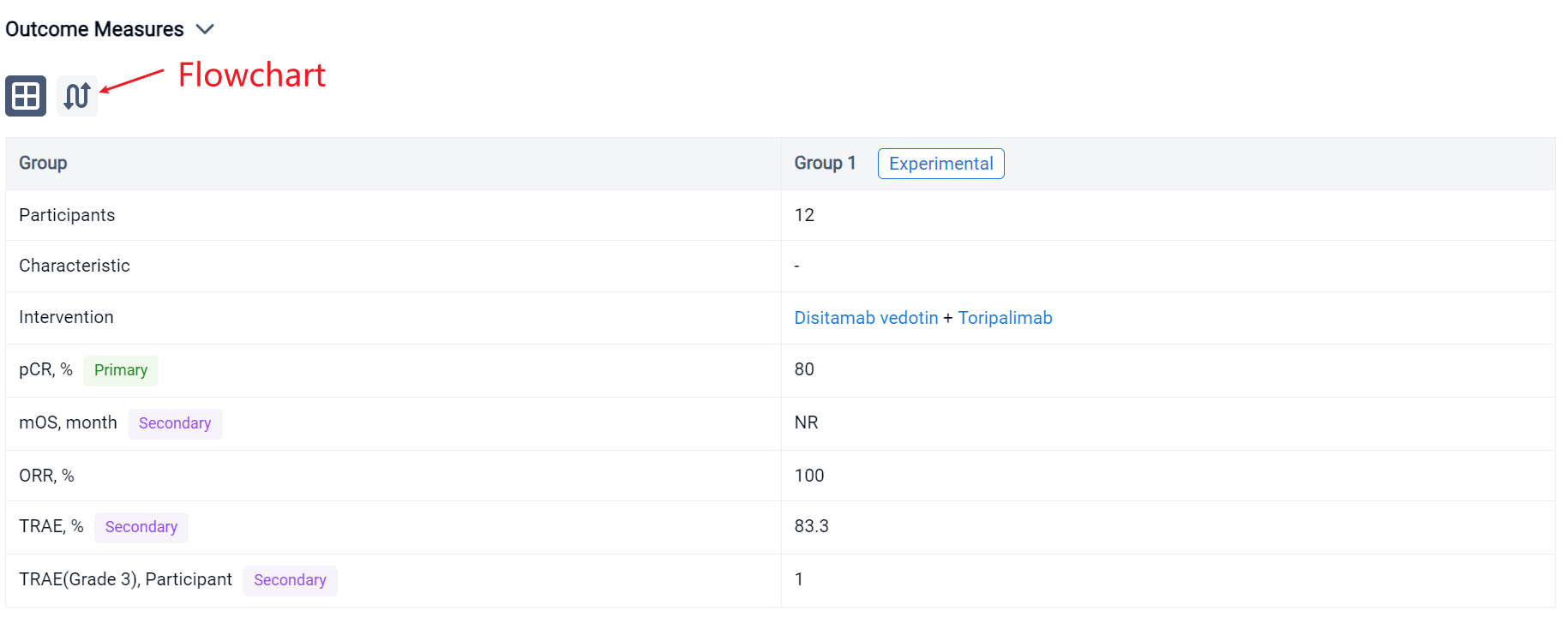
The result showed that from July 2023 to Sep 2023, 12 patients were enrolled. The median age was 68 (55-82) year. By the data cut off in August 2023, the primary end point pCR was 80% (8/10). Median follow-up time was 4.2 (2.2-8.45) month. The median overall survival (OS) was not reached,ORR was100%. In terms of safety, the treatment-related adverse events (TRAEs) of any grade occurred in ten patients (83.3%), and the most common TRAEs were nausea (58.3%), followed by alopecia (33.3%), diarrhea (16.7%) and Cardiac tachycardia (8.3%). most of which are grade 1-2. Grade 3 TRAE is Cardiac tachycardia and occurred in one patient. No grade 4 and 5 TRAEs were observed.
How to Easily View the Clinical Results Using Synapse Database?
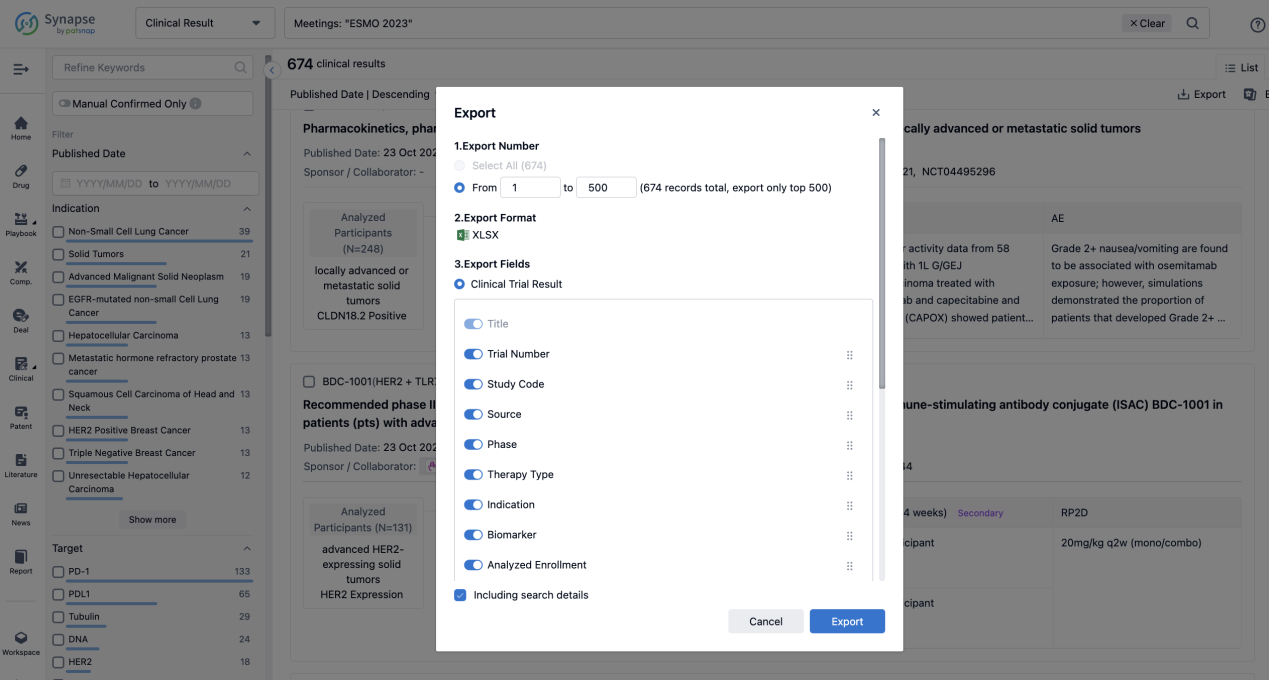
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
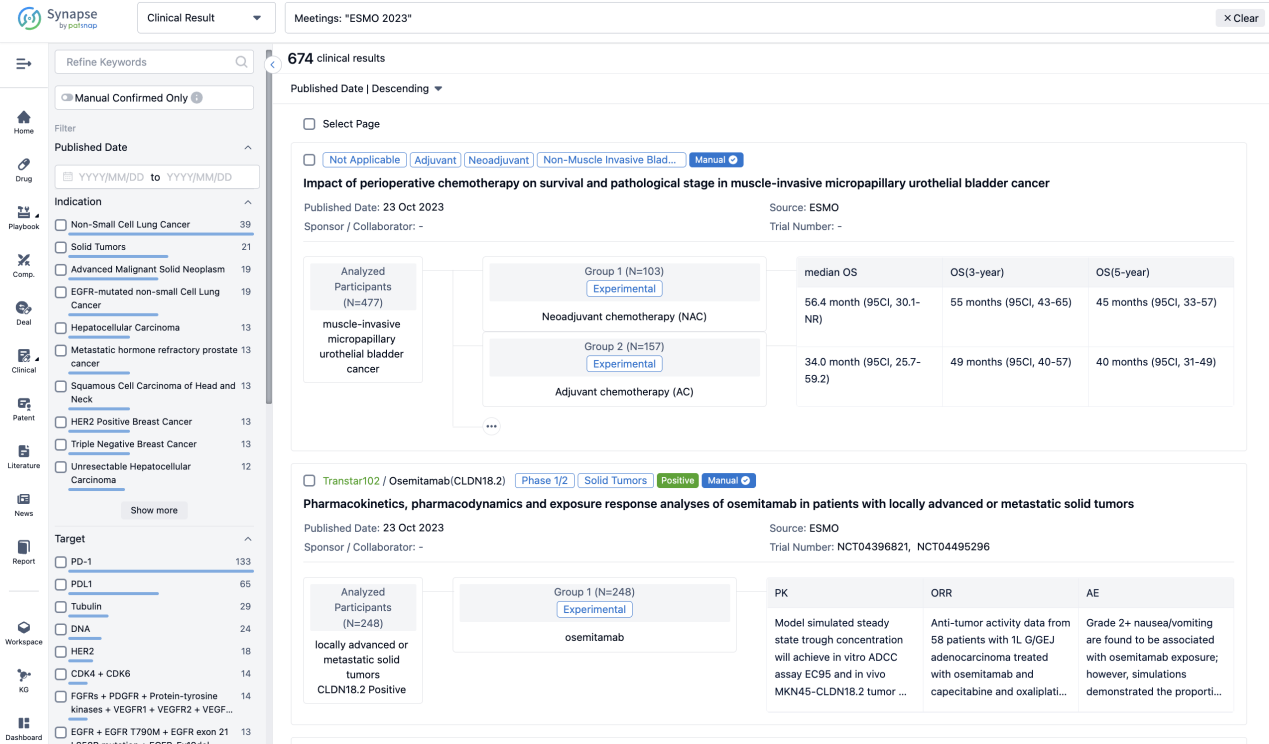
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!